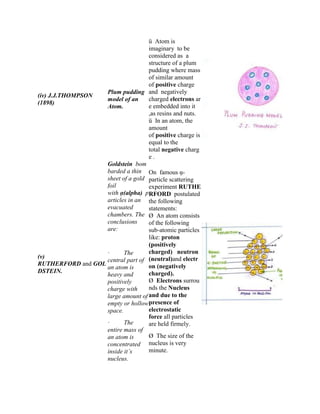
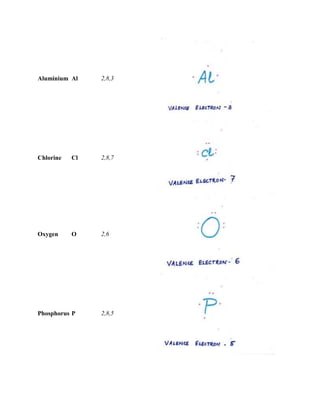
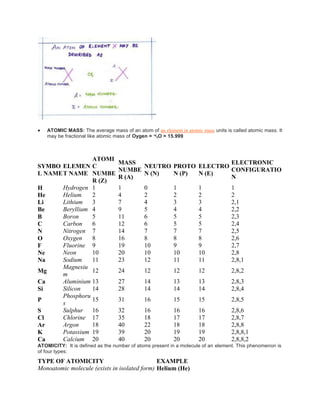
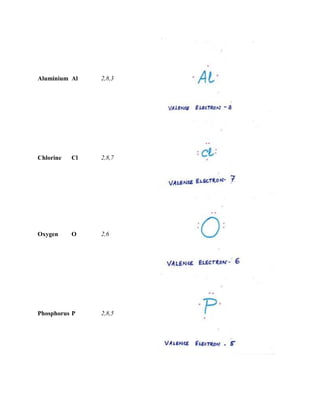
The document discusses the atomic structure, including the historical development of atomic models by various scientists such as Dalton, J.J. Thompson, Rutherford, and Bohr. It outlines the fundamental concepts of atoms, subatomic particles, atomic number, and mass number, as well as the concept of atomicity and valence electrons. Additionally, it highlights different elemental configurations and their properties.