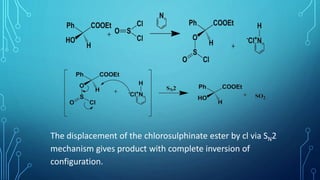
The document discusses the Sni (substitution nucleophilic internal) mechanism. In the Sni mechanism, part of the leaving group attacks the substrate as it detaches from the rest of the leaving group. This allows the reaction to occur with retention of configuration and no neighboring group participation. The document also discusses factors that affect the Sni mechanism like solvent, substituents, and chloride ion concentration. Finally, it examines nucleophilic substitution mechanisms at vinyl carbons including tetrahedral, addition-elimination, and elimination-addition pathways.













![3. Elimination – addition mechanism:
The reaction does not proceed without Eto- . No simple
substitution Rate α [Eto] but not on [ArSH]. Both lead to
retention. Since both are anti addition and elimination.](https://image.slidesharecdn.com/vinylcarbon-230307144829-68990683/85/Vinyl-carbon-pptx-15-320.jpg)


