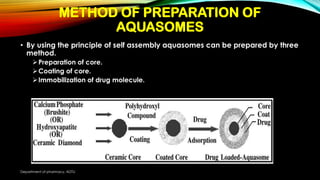
This document discusses Aquasomes, which are nanoparticle carrier systems composed of a central solid nanocrystalline core coated with polyhydroxy oligomers onto which drug molecules can be adsorbed. Aquasomes are spherical particles 60-300nm in size that are used for targeted drug and antigen delivery. They are prepared through a self-assembly process involving the preparation of a ceramic core, coating the core with carbohydrates, and then immobilizing a drug molecule onto the coated core. Aquasomes have properties such as preserving the integrity of biomolecules and avoiding clearance from the body. They can be characterized through techniques like SEM, TEM, FT-IR, and XRD. Potential applications of Aquasomes