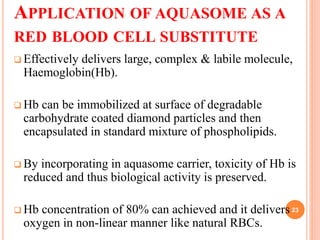
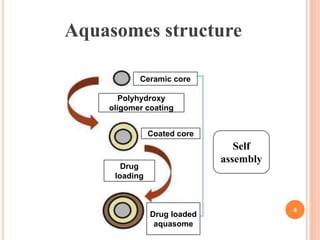
The document discusses aquasomes, which are self-assembled three-layered nanostructures that protect and preserve fragile biological molecules. It details their preparation process, which includes creating a ceramic core, coating it with carbohydrates, and immobilizing drugs. Applications of aquasomes include delivering vaccines, insulin, and as a red blood cell substitute, demonstrating their potential in drug delivery systems.











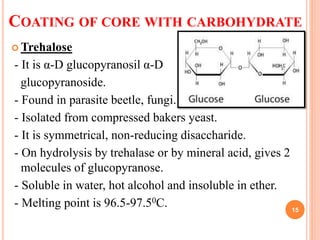
![COATING OF CORE WITH CARBOHYDRATE
Pyridoxal-5-phosphate
- Prepared by action of phosphorous
oxychloride on pyridoxal in
aqueous solution and by phosphorylation of
pyridoxamine with
100% H3PO4 followed by oxidation.
- It is colourless in acid solution and bright yellow in
alkaline solution.
- On oxidation with Hydrogen peroxide in alkaline
solution gives [(2-methyl-3,4-dihydroxy-5-
pyridyl)methyl] phosphoric acid.
- It gives negative 2,6-dichloroquinone chlorimide test.
14](https://image.slidesharecdn.com/aquasomes-200702103213/85/Aquasomes-14-320.jpg)