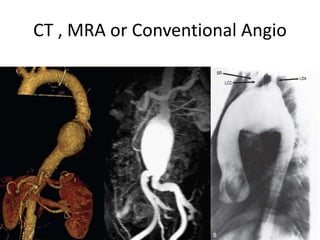
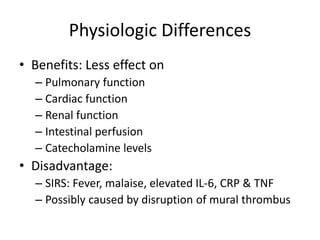
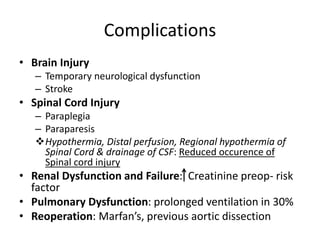
1. The document discusses various methods for evaluating aortic aneurysms including CT, MRI, and aortography. CT is useful for determining size and details of the aneurysm but risks radiation exposure, while MRI does not require contrast but has longer scan times and other limitations.
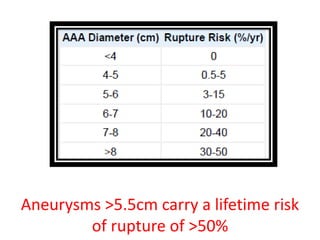
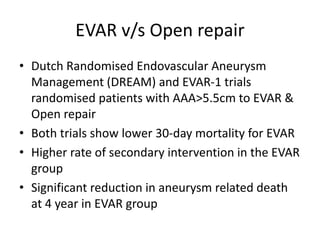
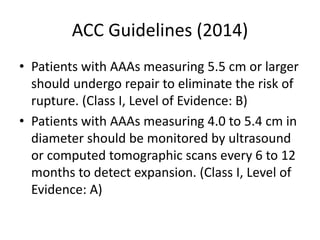
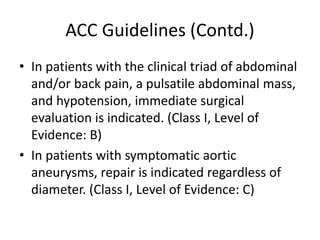
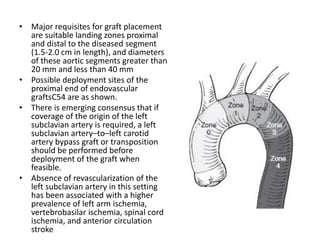
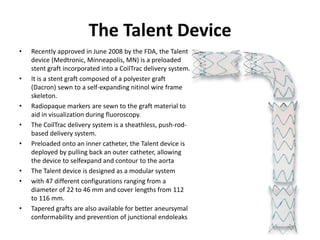
2. Factors such as aneurysm size greater than 5.5cm, shape, wall thickness, and presence of thrombus impact the risk of rupture. Open or endovascular repair options are discussed based on risk factors. Endovascular repair has lower short-term mortality but higher reintervention rates.
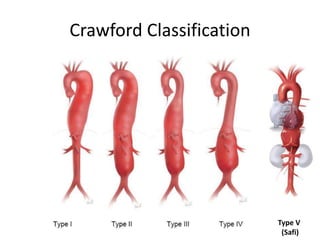
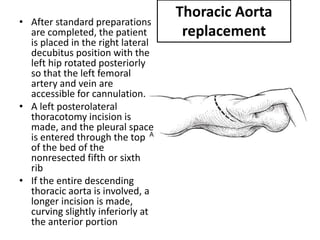
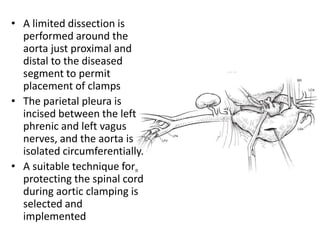
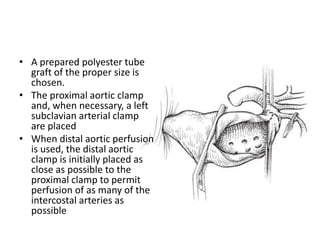
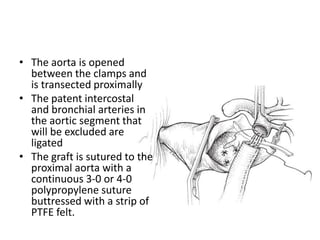
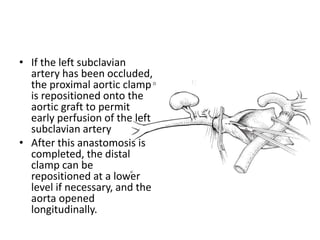
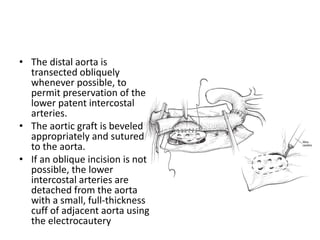
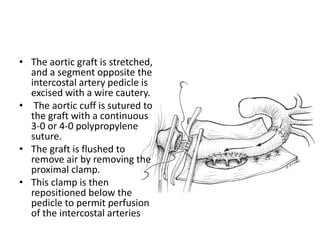
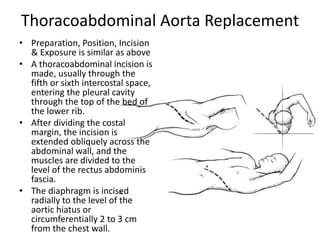
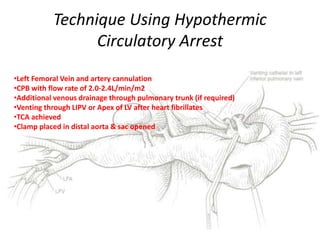
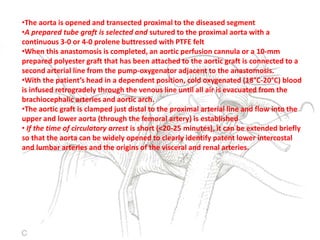
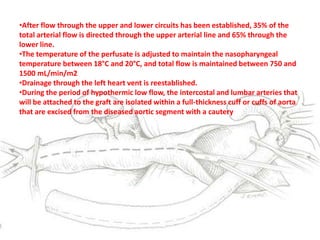
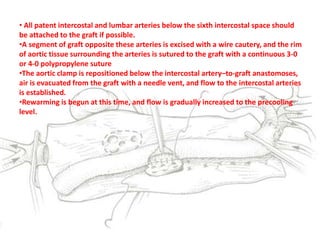
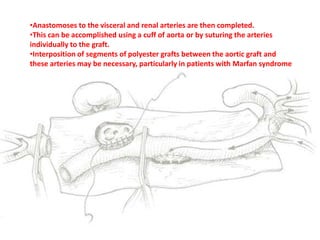
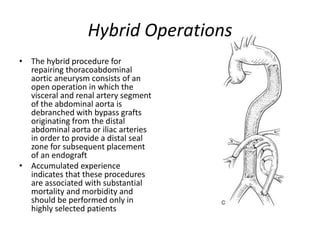
3. Details are provided on preparations, techniques, and steps for open surgery to replace the thoracic or thoracoabdom