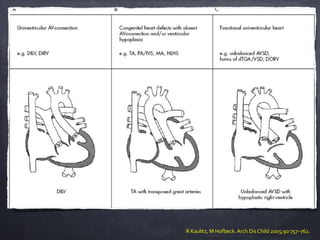
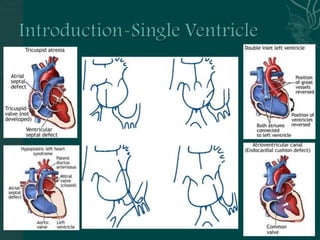
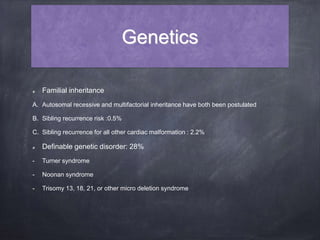
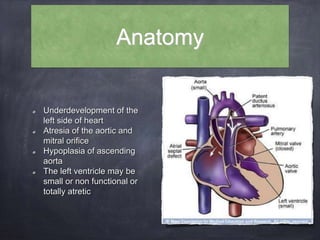
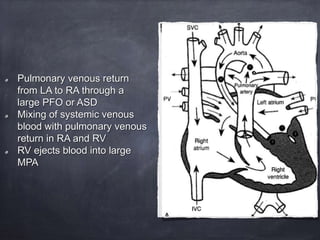
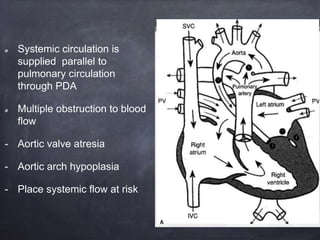
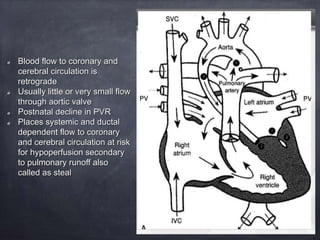
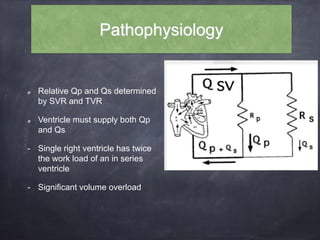
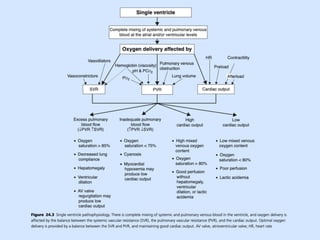
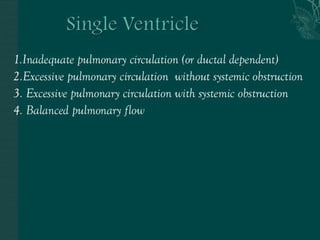
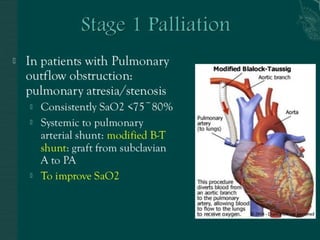
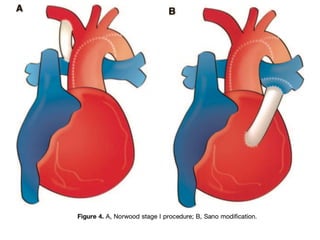
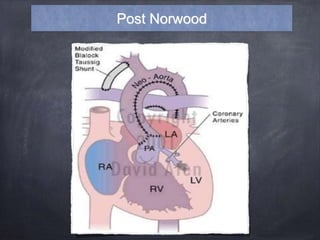
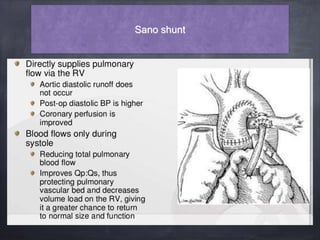
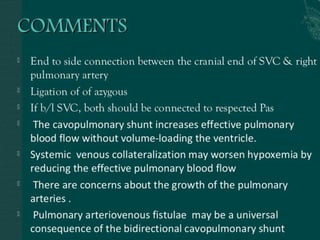
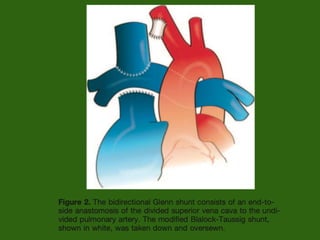
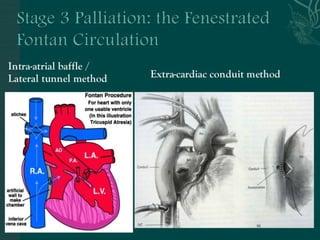
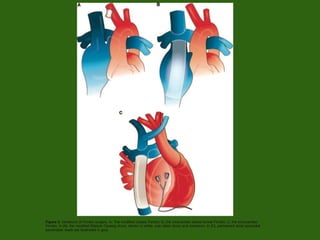
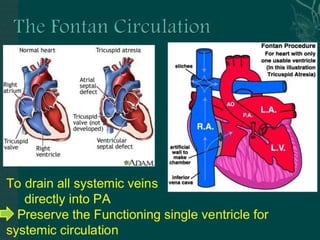
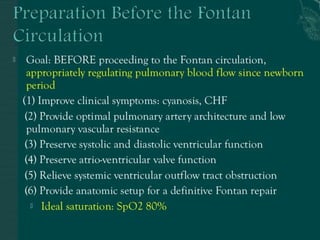
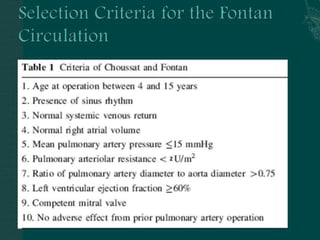
Single ventricle physiology refers to congenital heart defects where the entire atrioventricular junction connects to a single ventricular chamber. This includes conditions like double inlet left ventricle (DILV), tricuspid atresia, and unbalanced atrioventricular septal defects. The underlying embryology is not fully understood but is thought to involve limitations on inflow or outflow of the left ventricle. Initial management focuses on optimizing pulmonary and systemic blood flow without overloading the single ventricle. Surgical options have evolved from supportive care to staged reconstruction like the Norwood procedure and subsequent Fontan operations.