Pulmonary artery banding (PAB) is a palliative surgical technique used to reduce pulmonary blood flow in infants with congenital heart defects. It involves placing a band around the pulmonary artery to create stenosis and decrease blood flow to the lungs. PAB is used as an initial intervention for defects causing pulmonary overcirculation to prevent congestive heart failure and pulmonary hypertension before a definitive repair. It is also used to prepare the left ventricle in some patients with transposition of the great arteries prior to later procedures. The goal of PAB is to reduce pulmonary pressures and improve systemic circulation. It remains an important technique for staged surgical treatment of certain congenital heart conditions.


![History of the Procedure
• The first description of pulmonary artery banding (PAB) in
the literature was a report by Muller and Dammann at the
University of California, Los Angeles (UCLA) in 1951.
• In this report, Muller and Dammann described palliation by
the "creation of pulmonary stenosis" in a 5-month-old
infant who had a large ventricular septal defect (VSD) and
pulmonary overcirculation.
• Following this report, multiple studies were published
demonstrating the effectiveness of this technique in infants
with congestive heart failure (CHF) caused by large VSDs,
complex lesions (eg, atrioventricular canal [AVC] defects),
and tricuspid atresia.
• Although the use of PAB has declined, it remains an
essential technique for comprehensive surgical treatment
in patients with congenital heart disease. PAB is a palliative
but not a curative surgical procedure.](https://image.slidesharecdn.com/pabandingnew-180313181407/85/Pa-banding-new-4-320.jpg)




![Contraindications
• Patients who have single ventricle defects in which the
aorta arises from an outflow chamber (eg, double inlet
left ventricle [LV], tricuspid atresia with transposition of
the great arteries [TGA]) have the potential for
development of significant subaortic obstruction.
• Pulmonary artery banding (PAB) is contraindicated in
the presence of such obstruction and in patients who
are at high risk for such obstruction.
• The ventricular hypertrophy that develops in response
to PAB may cause rapid progression of subaortic
obstruction leading to a combination of both ventricles
having outflow tract obstruction and progressive
hypertrophy.](https://image.slidesharecdn.com/pabandingnew-180313181407/85/Pa-banding-new-16-320.jpg)


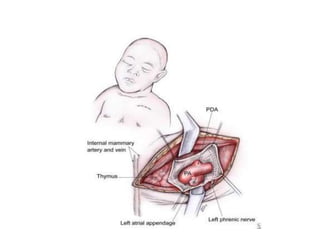

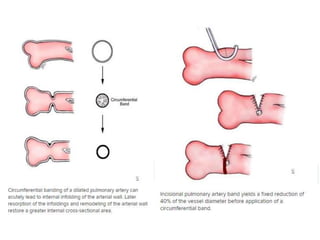
![• The MPA and aorta are exposed, and the band is
prepared for placement.
• The estimated band circumference is marked on the
umbilical tape with fine sutures according to the
Trusler formula.
• PAB circumference in patients with noncyanotic
nonmixing lesions (eg, ventricular septal defect [VSD])
is 20 mm + 1 mm/kg body weight. For patients with
mixing lesions (eg, D-transposition of the great arteries
[TGA] with VSD), the formula is 24 mm + 1 mm/kg
body weight. In patients with single ventricles in whom
the Fontan procedure is planned, an intermediate
circumference of 22 mm + 1 mm/kg body weight is
preferred.](https://image.slidesharecdn.com/pabandingnew-180313181407/85/Pa-banding-new-23-320.jpg)

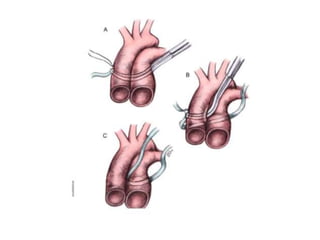

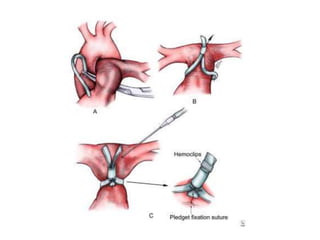

![• An adequate atrial communication should be
confirmed preoperatively in patients with single
ventricle physiology, including transposition with VSD
complexes. A balloon atrial septostomy is useful in
these situations.
• Kotani and colleagues measured intraoperative aortic
blood flow using a Transonic flow probe and found that
aortic blood flow increased by approximately 40% after
successful PAB. Their data suggested that higher pre-
PAB Qp/Qs (pulmonary [Qs]-systemic [Qp] blood flow
ratio) predicted a higher percentage increase in aortic
flow.
• Three patients with less than a 20% increase in aortic
blood flow died, required re-PAB, or developed
ventricular dysfunction.](https://image.slidesharecdn.com/pabandingnew-180313181407/85/Pa-banding-new-31-320.jpg)

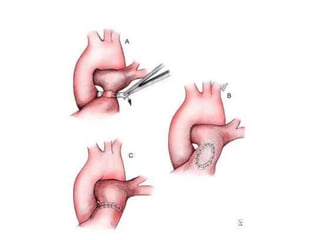
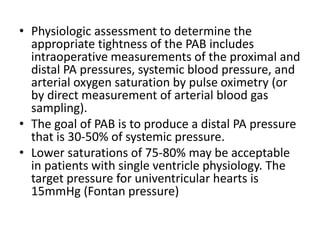
![• PAB takedown is usually performed at the time of the
intracardiac repair through a median sternotomy.
Generally, the repair is completed first and the PAB
removal is performed at the end of the procedure.
• The band is dissected free from surrounding scar tissue
and removed. The area of banding usually remains
stenotic and requires repair.
• This repair can be achieved by resection and end-to-
end anastomosis of the proximal and distal MPA or by
vertical incision of the MPA followed by pericardial (or
polytetrafluoroethylene [PTFE]) patch repair of the
arteriotomy
• The repair must ensure relief of any branch PA stenosis
that may exist as a consequence of the PAB.](https://image.slidesharecdn.com/pabandingnew-180313181407/85/Pa-banding-new-33-320.jpg)