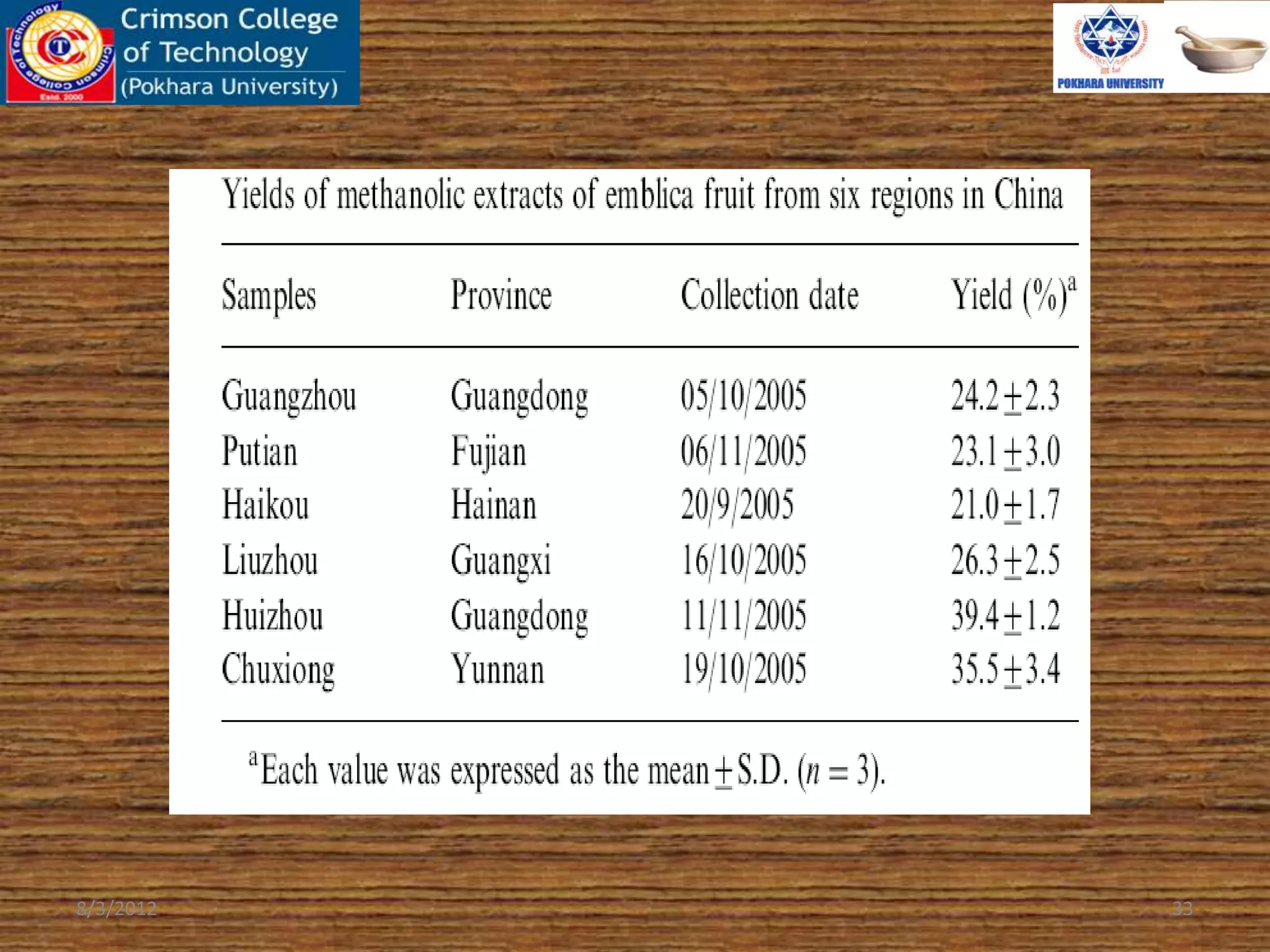
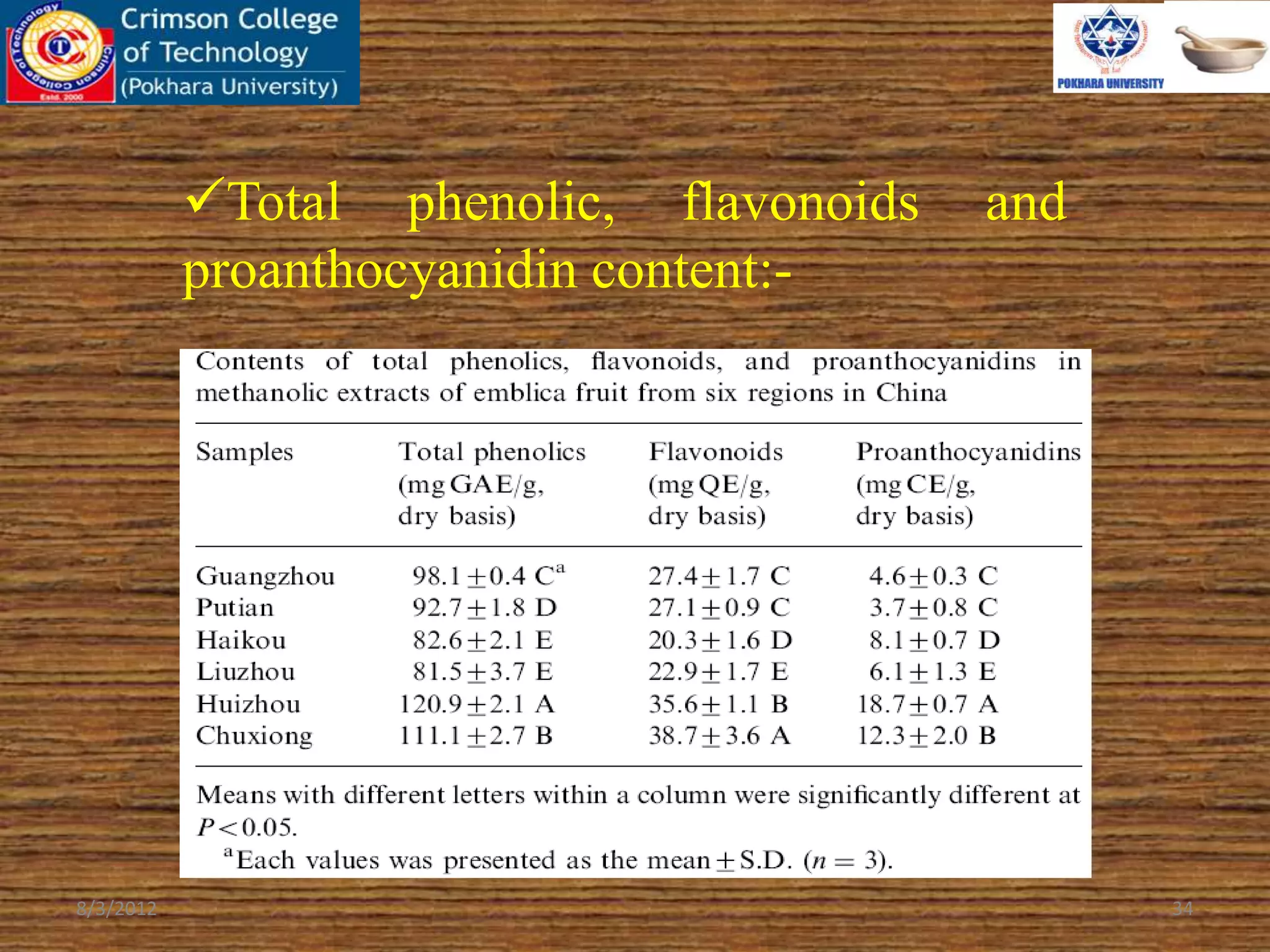
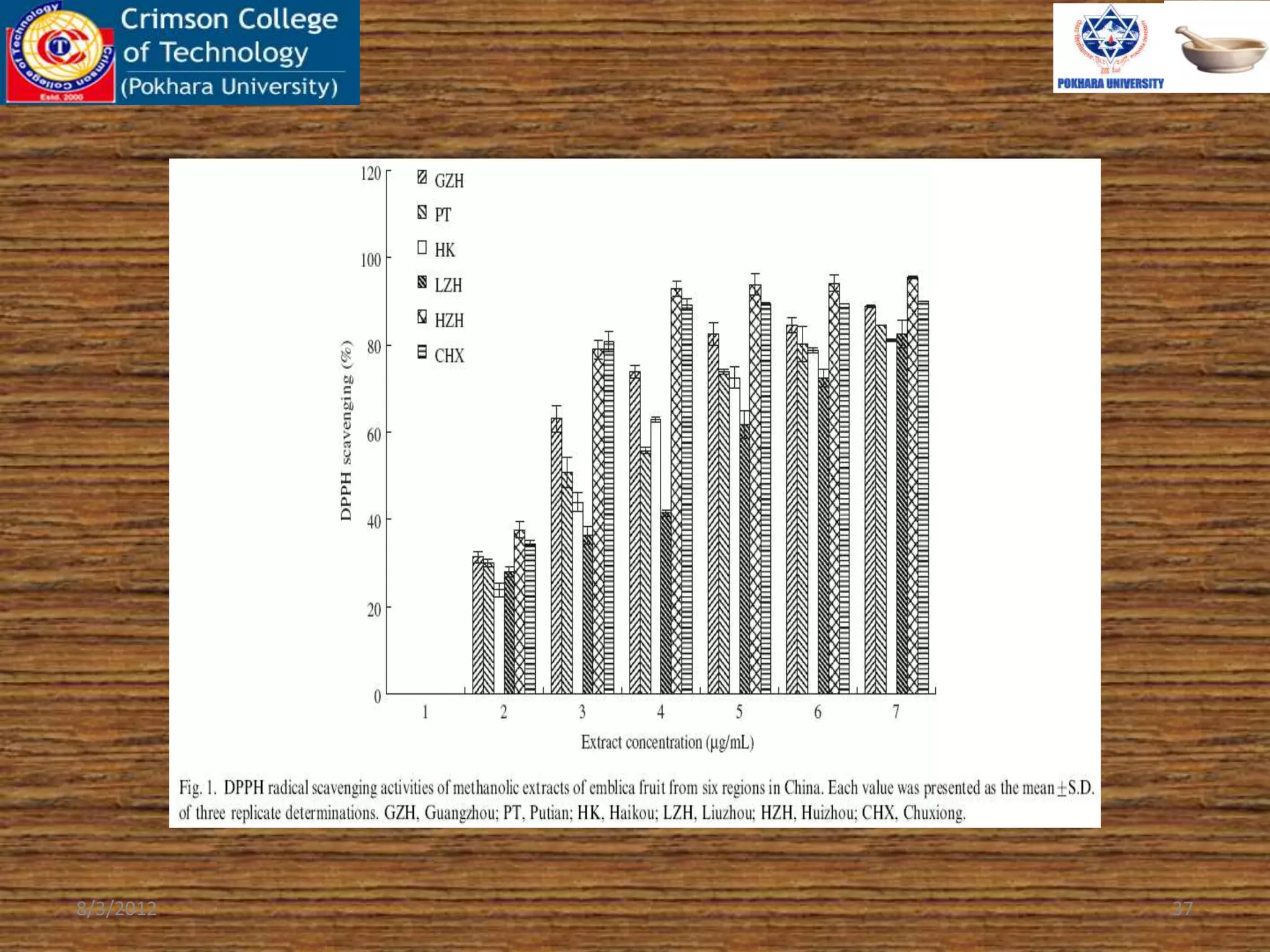
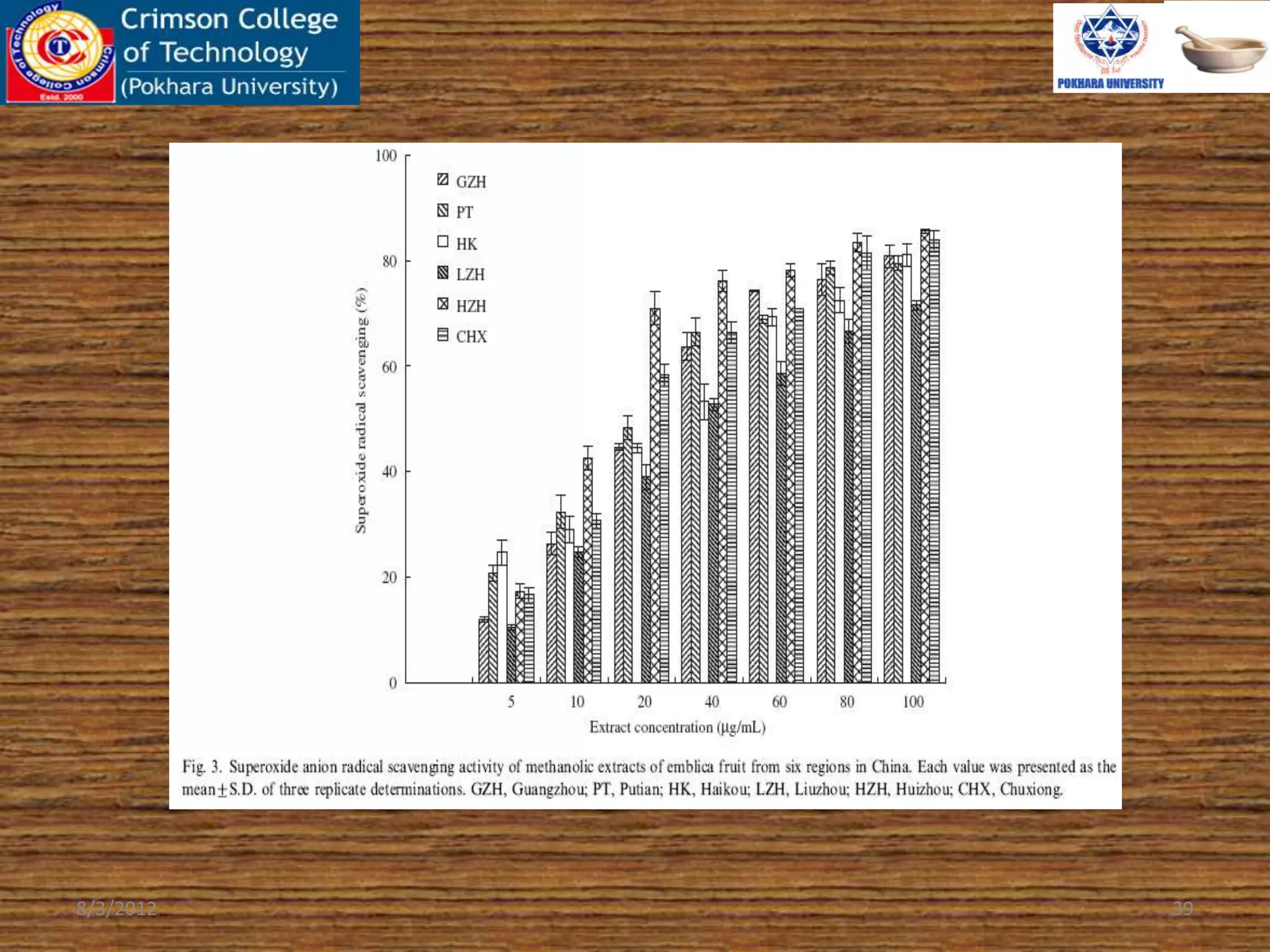
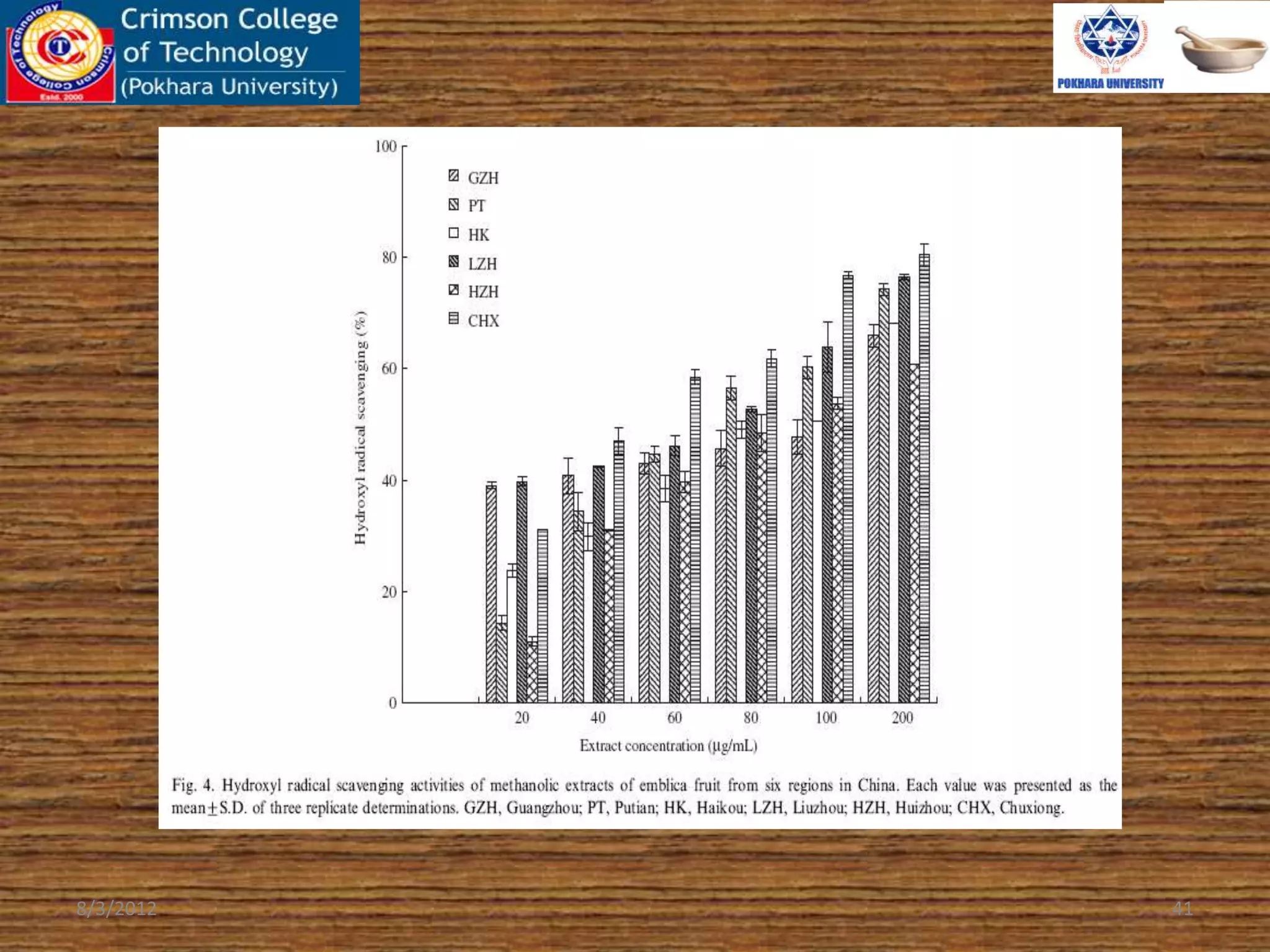
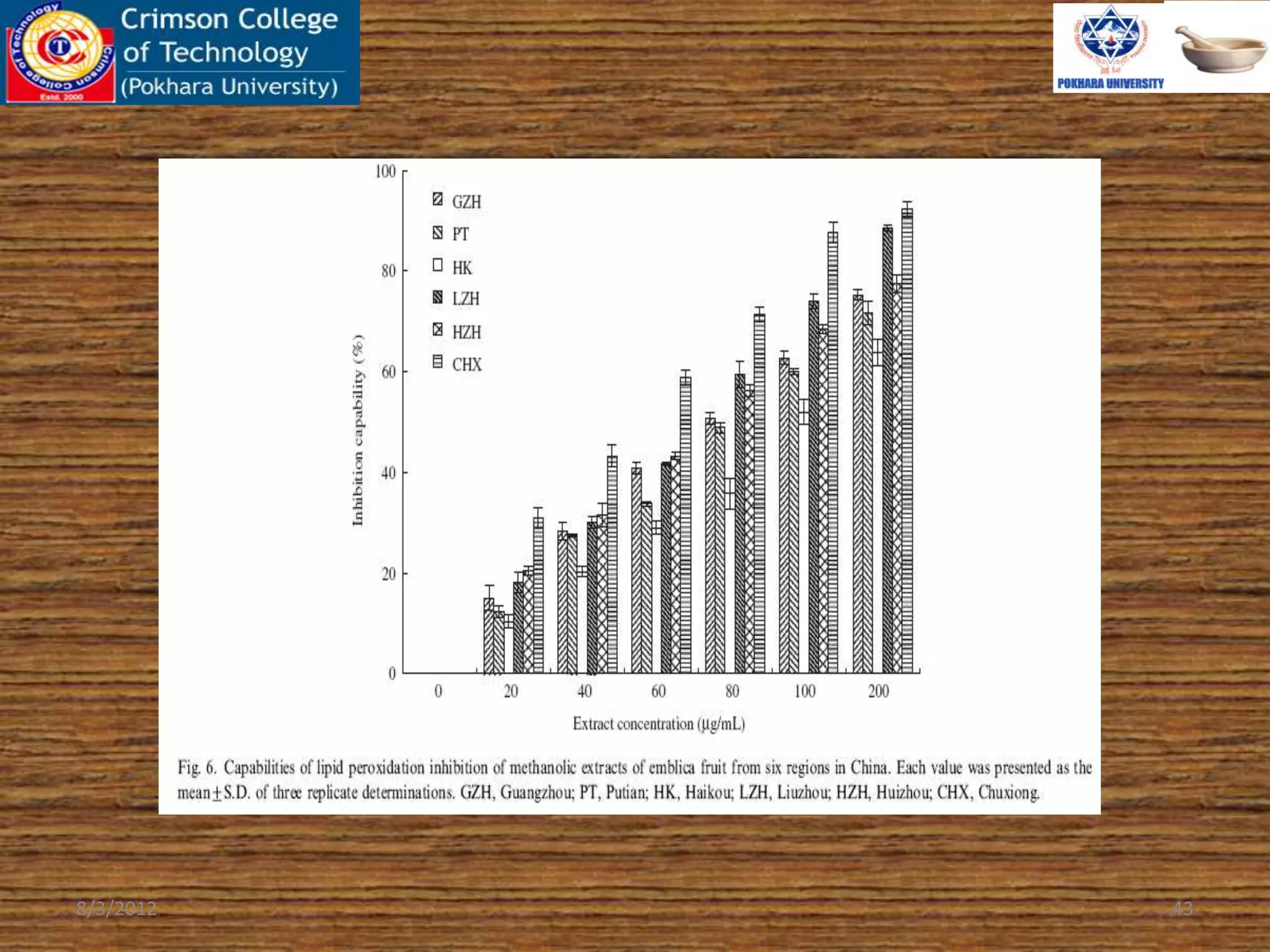
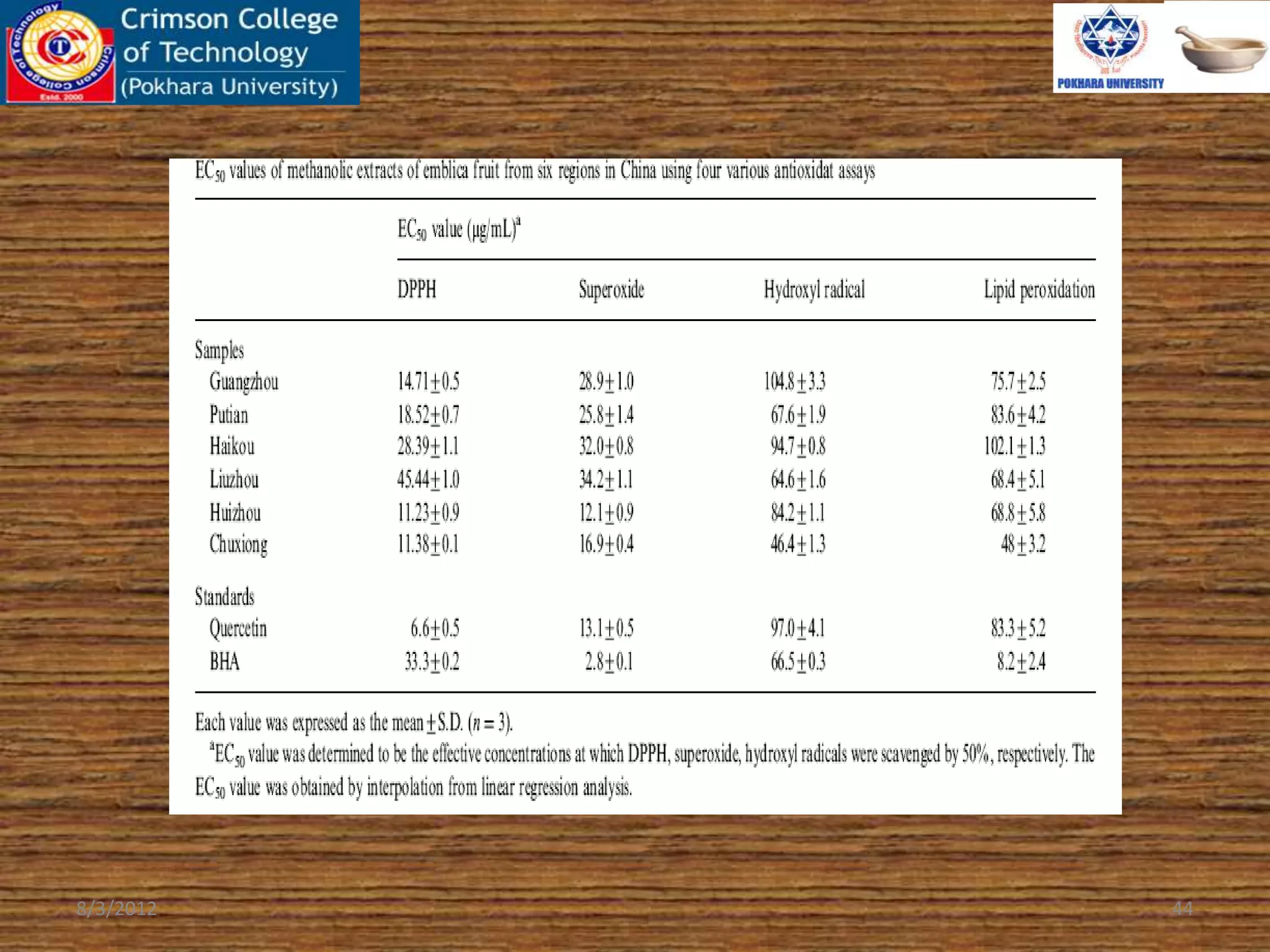
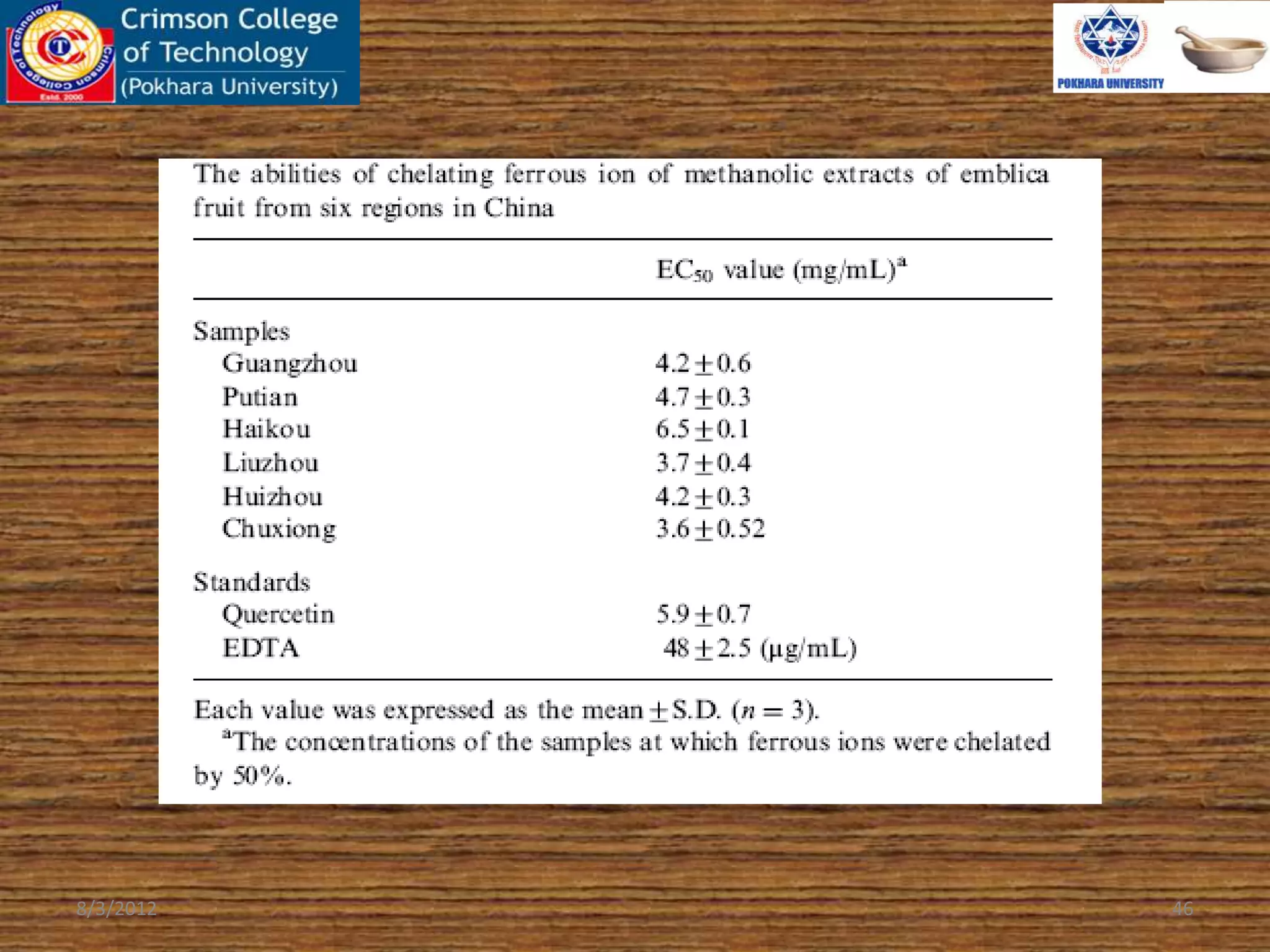
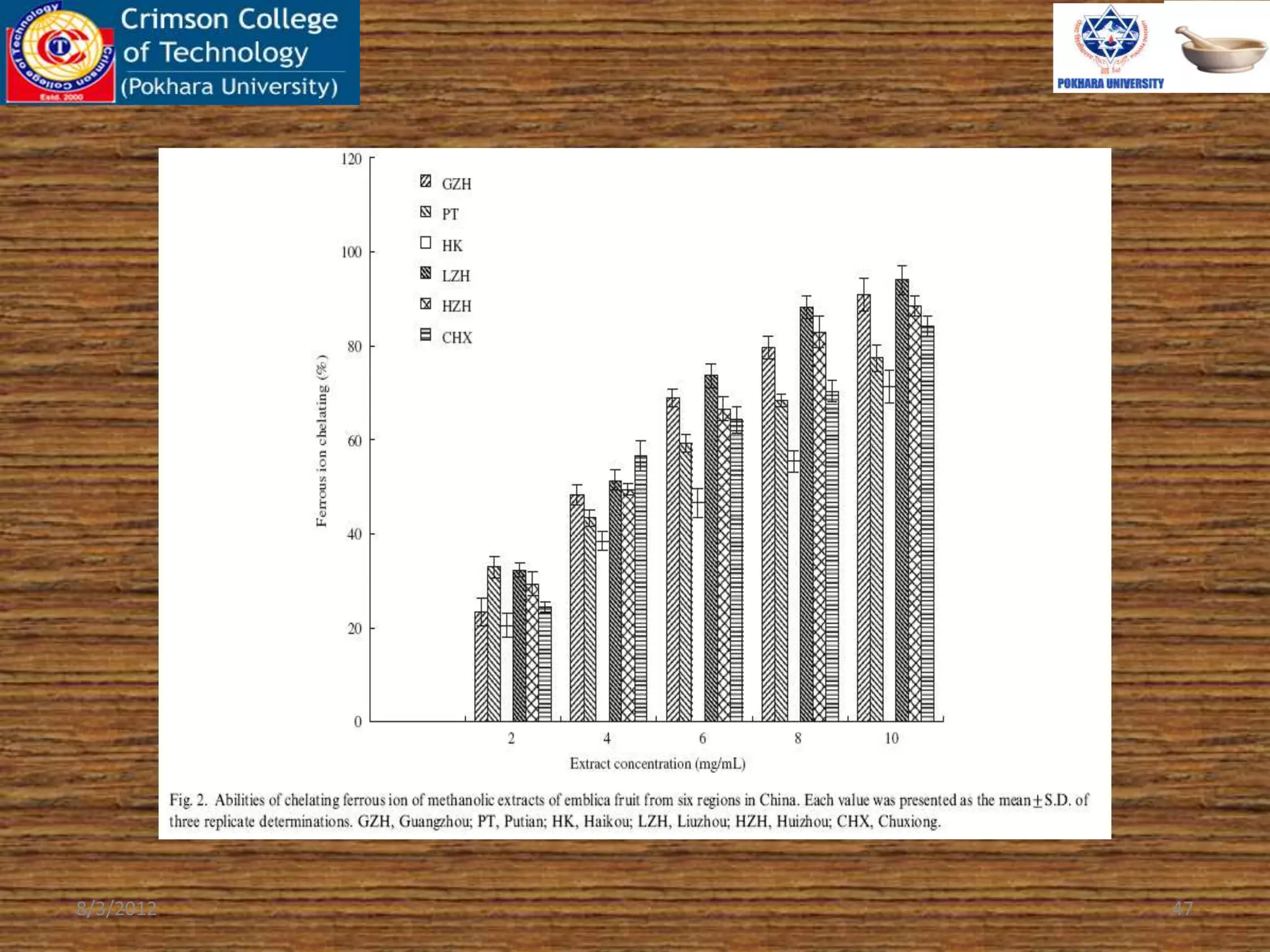
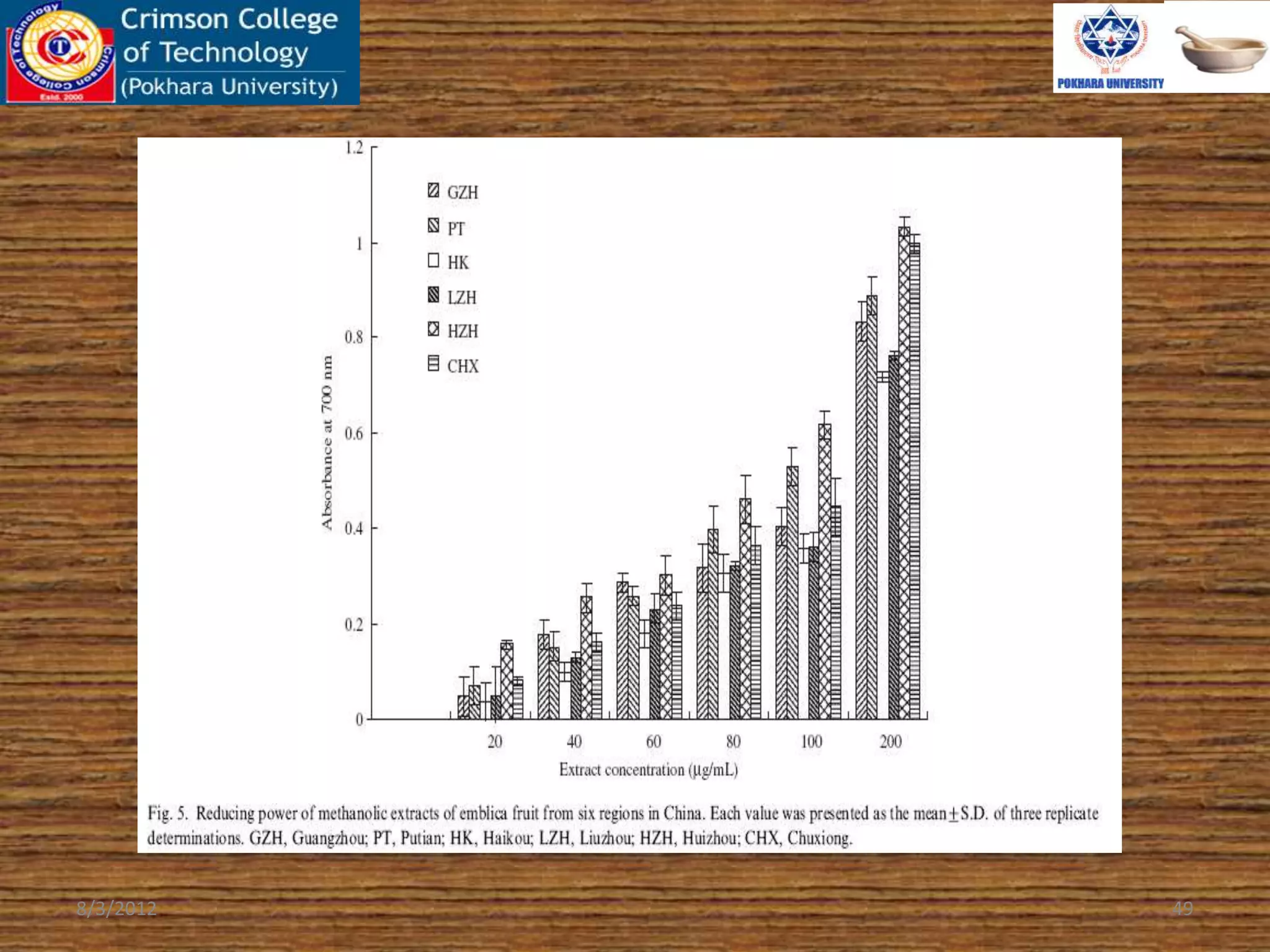
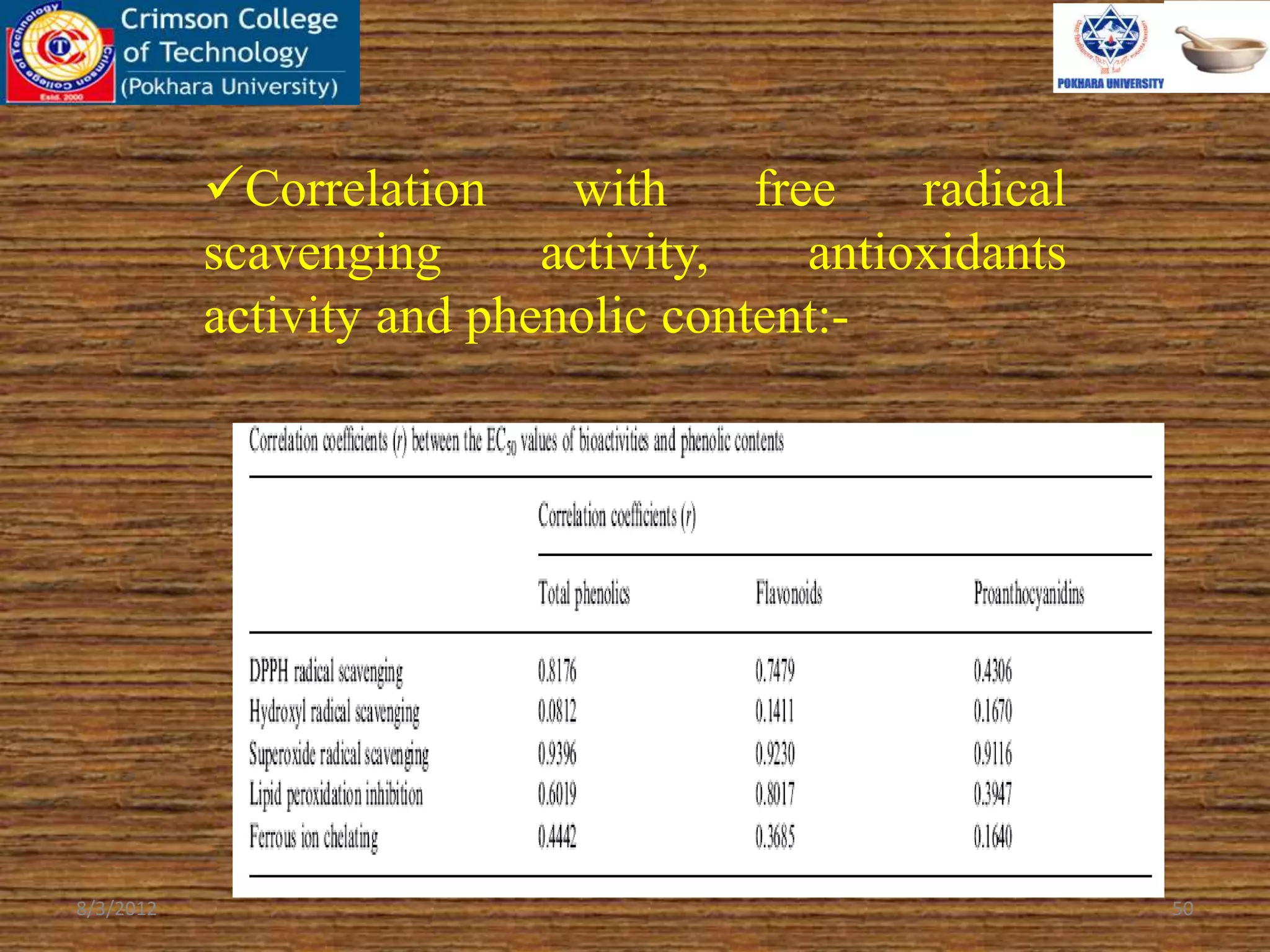
The document presents a study on the antioxidant activity of methanolic extracts from emblica fruit (Phyllanthus emblica L.) collected from six different regions in China. It details the methods for extracting the fruit, assessing total phenolic, flavonoids, and antioxidant activities, and discusses the significant variations in bioactive compounds based on the fruit's origin. The findings indicate that huizhou sample displays the highest antioxidant potential, suggesting the fruit's utility in food, cosmetic, and pharmaceutical applications.