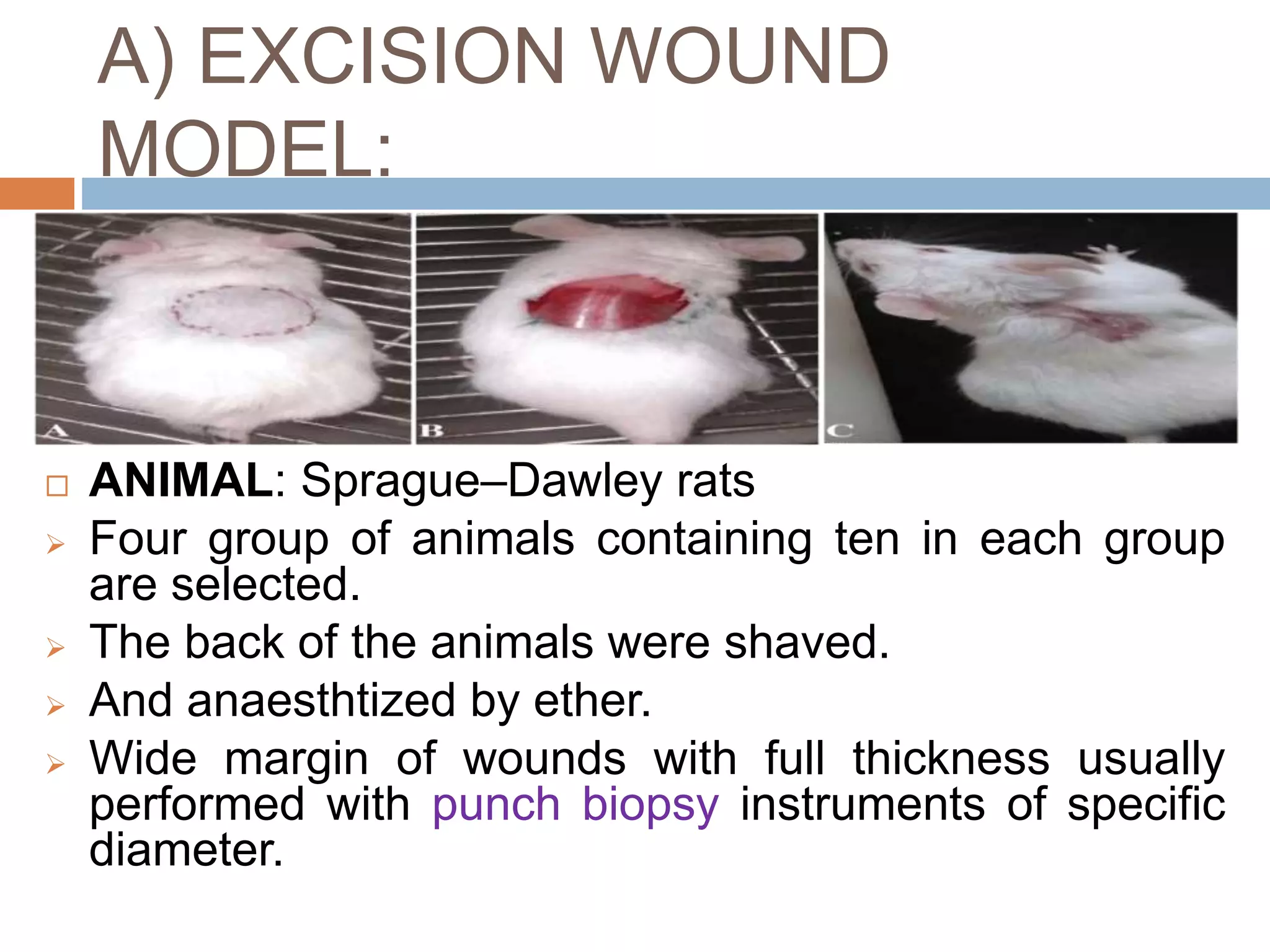
1) Various in vivo models are used to evaluate wound healing and hepatoprotective activity, including excision wounds, incision wounds, and burn wounds in rats.
2) Parameters like wound contraction, epithelization time, tensile strength and histopathology are measured to assess wound healing.
3) Hepatoprotective activity is evaluated by pre-treating animals with the test substance before inducing liver damage using toxins like CCl4, D-galactosamine, or paracetamol. Liver function is then assessed through serum enzymes and histopathology.