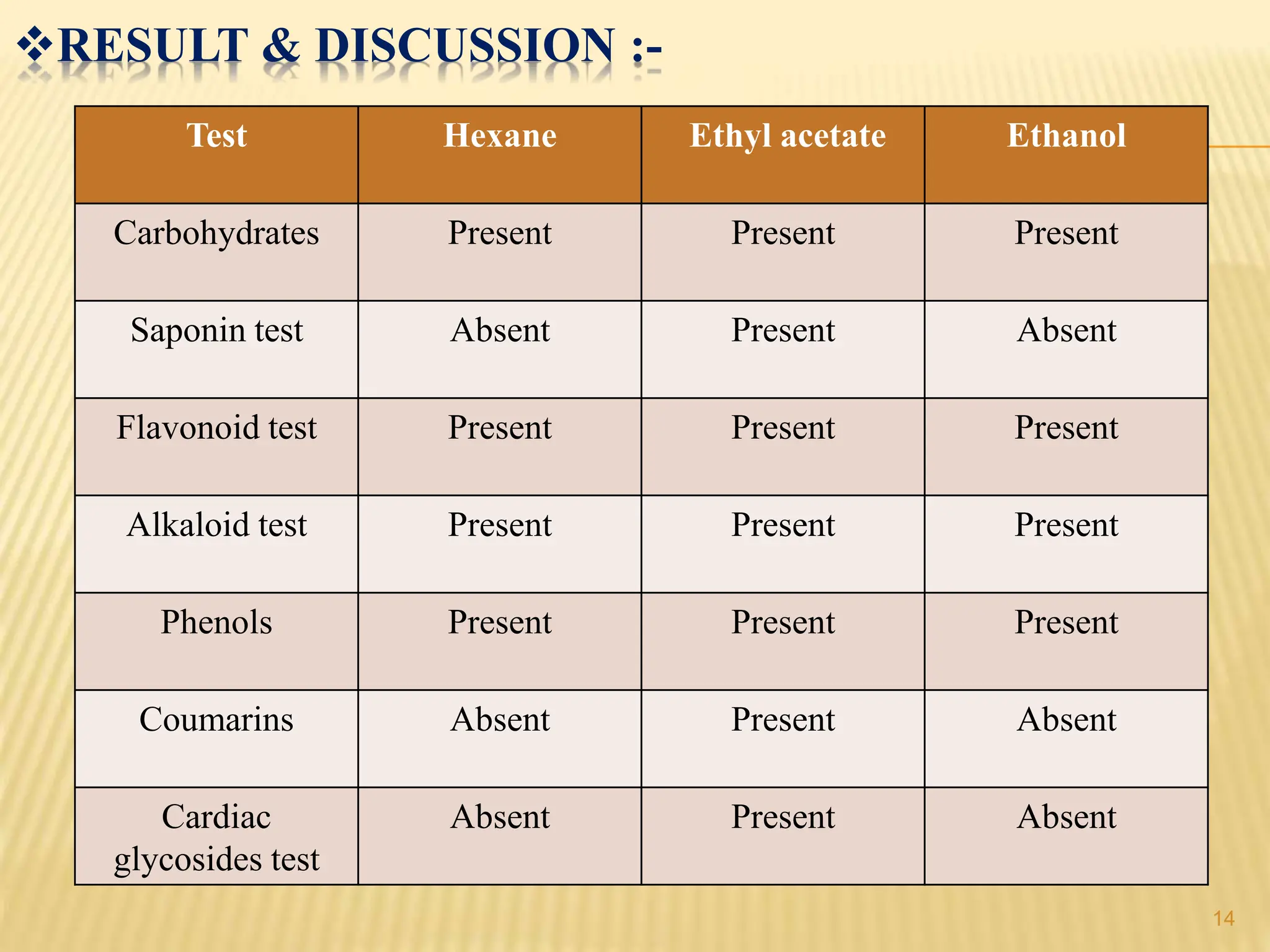
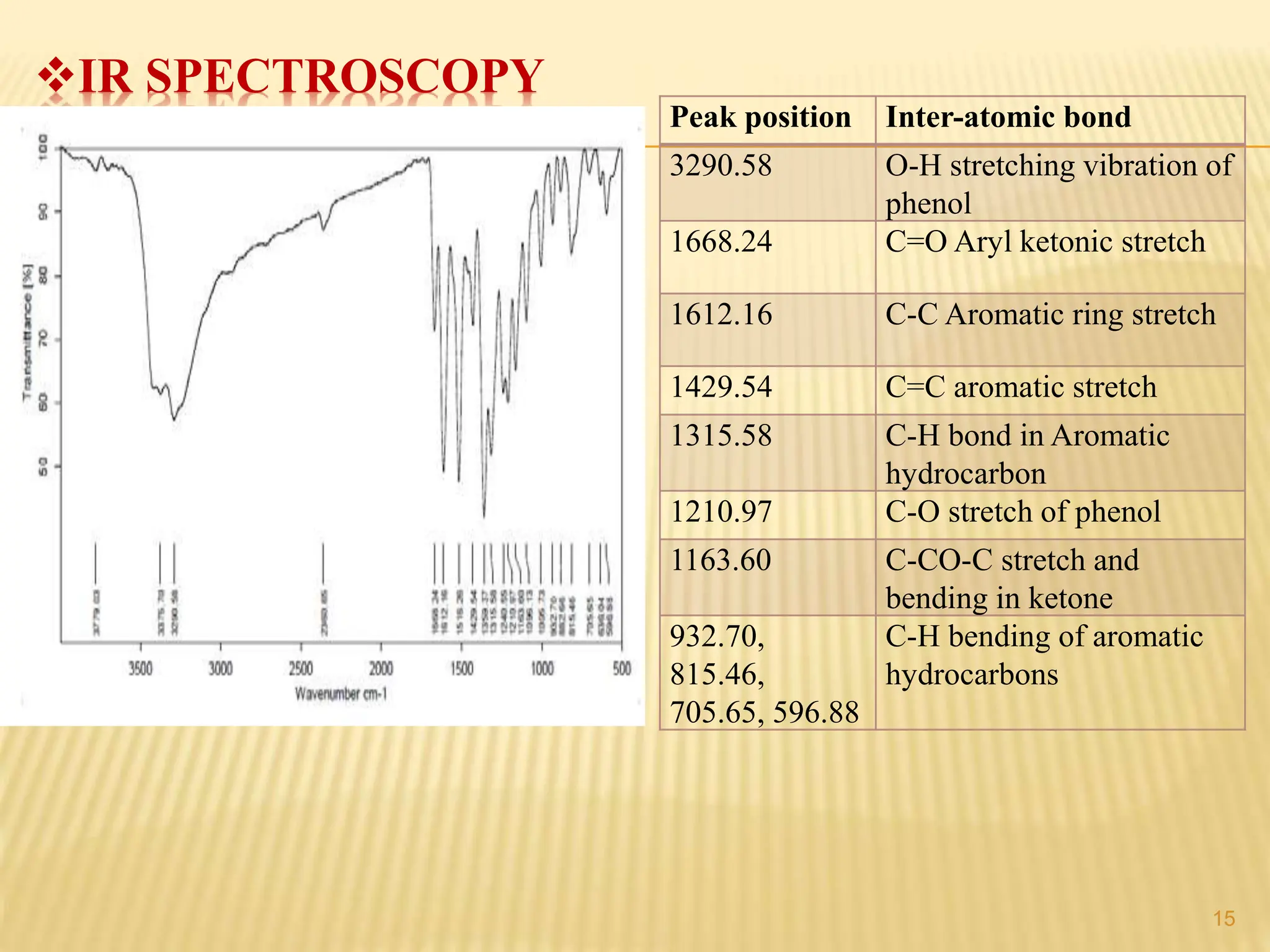
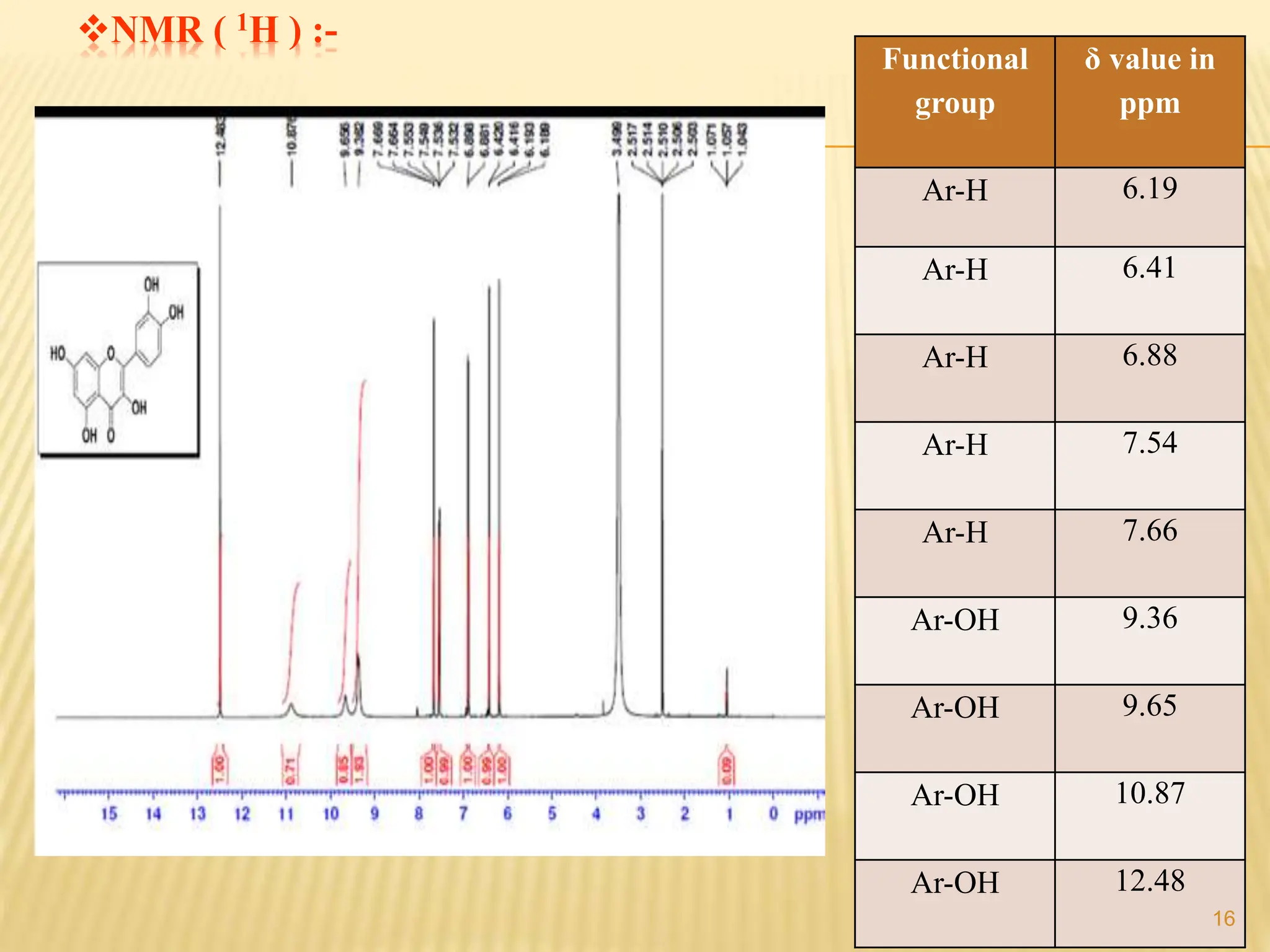
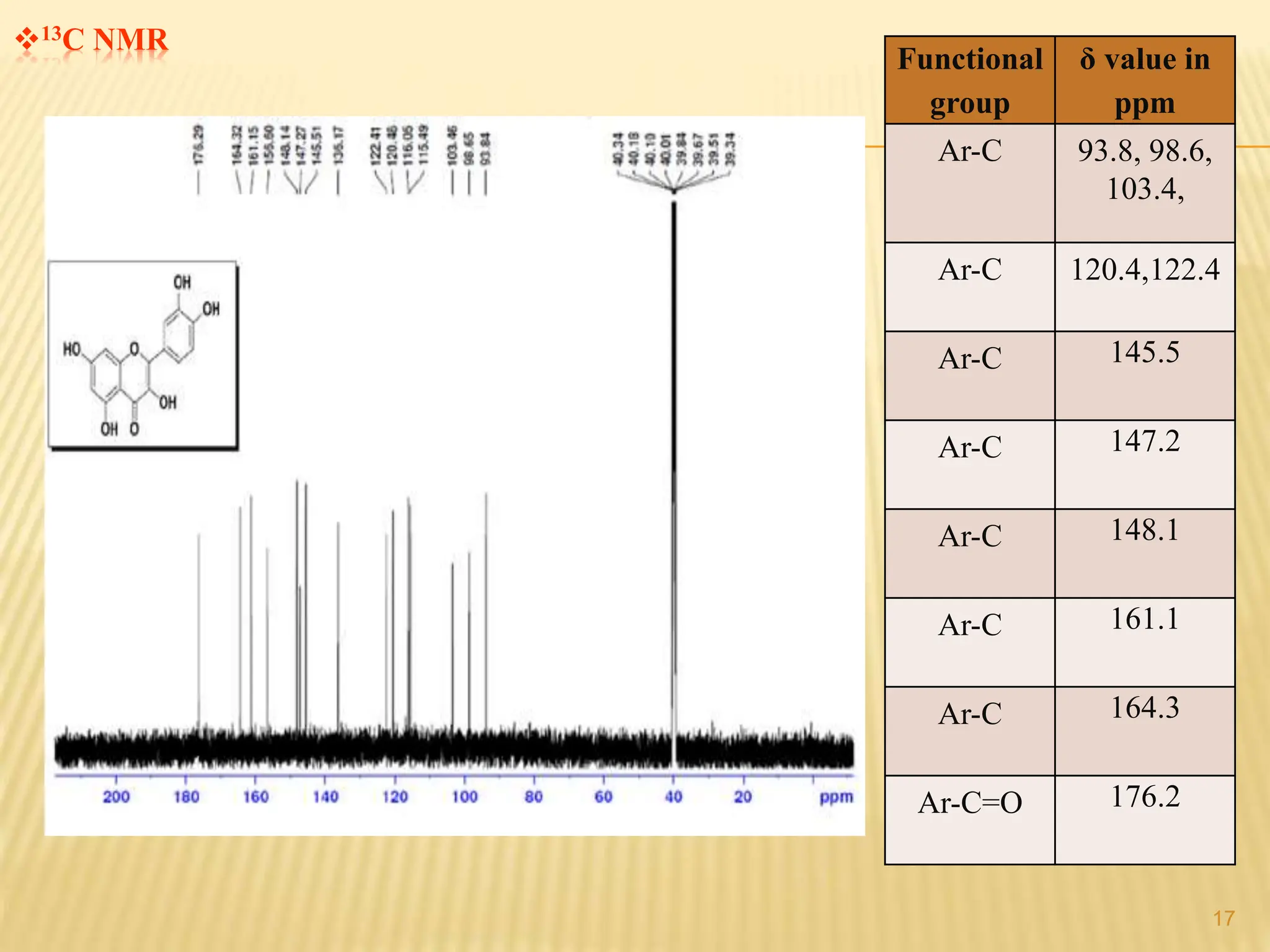
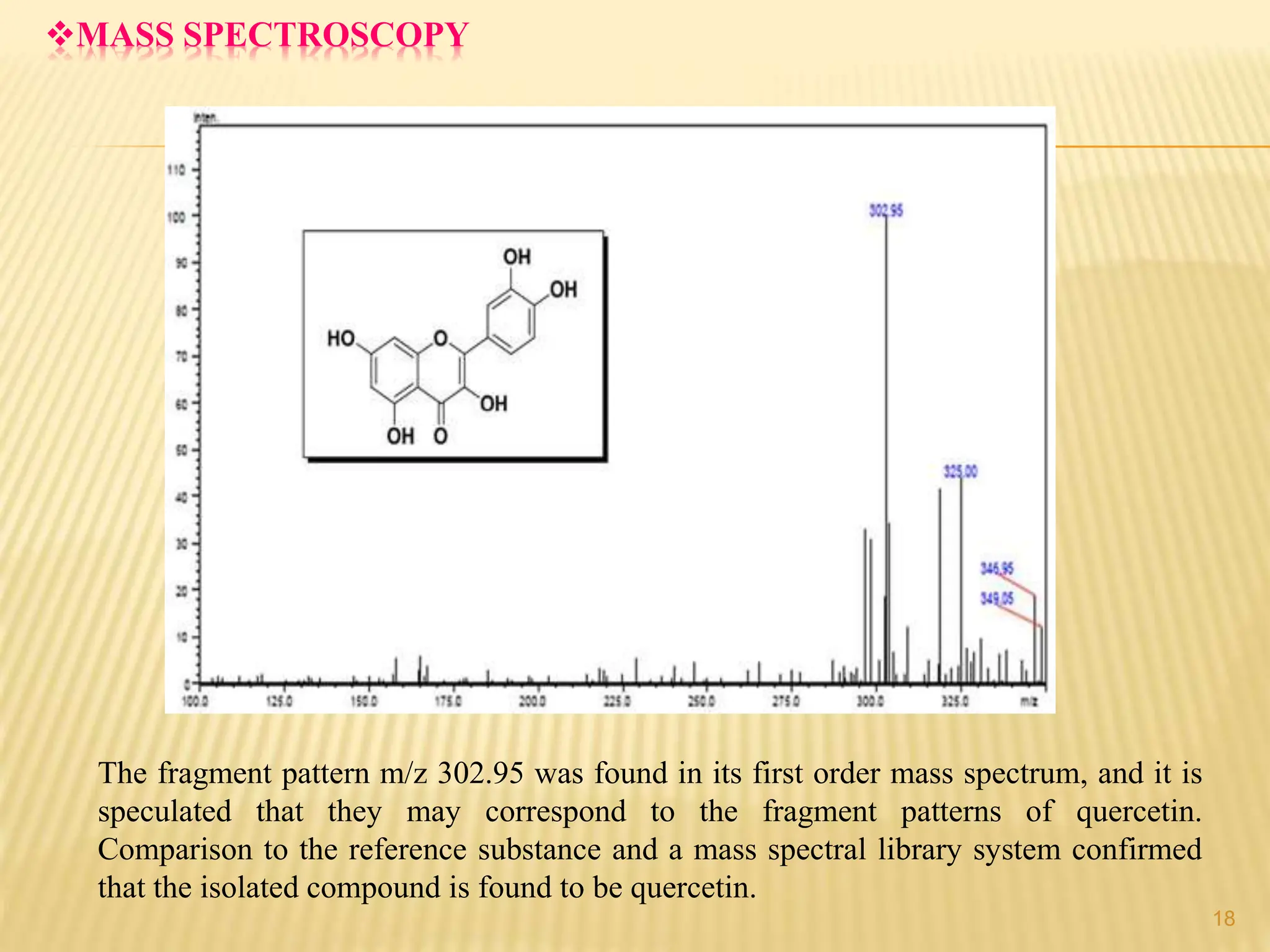
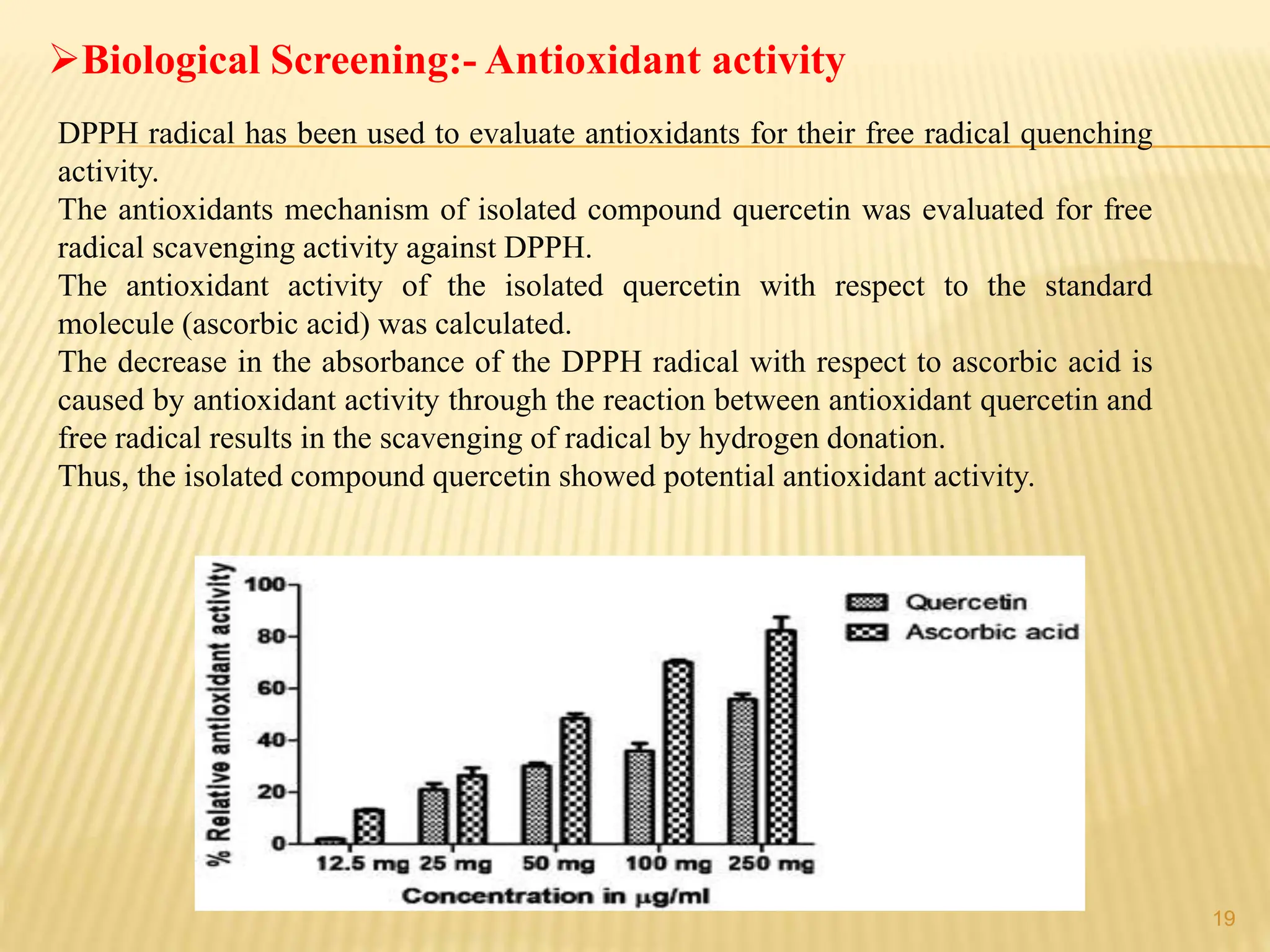
The study focused on isolating the bioactive flavonoid quercetin from the leaves of Trigonella foenum-graecum, using various solvents for extraction, with ethanol yielding the highest flavonoid content. The isolated quercetin was characterized through FT-IR, NMR, and mass spectroscopy, and demonstrated significant antioxidant activity in vitro. The research highlights a simple and efficient method for extracting bioactive compounds from plants, confirming the potential of quercetin as a natural antioxidant.








![DETERMINATION OF ANTIOXIDANT ACTIVITY
The free radical scavenging activity of isolated compound
quercetin was measured in vitro by 1,1-diphenyl-2-picrylhydrazyl
(DPPH) assay.
An aliquot of 0.5 ml of different concentration of sample solution
in methanol was mixed with 2.5 ml of 0.5 mM methanolic solution
of DPPH.
The mixture was shaken vigorously and incubated for 37 min in
the dark at room temperature.
The absorbance was measured at 517 nm using UV-Vis
spectrophotometer. Ascorbic acid was used as a positive control.
DPPH free radical scavenging ability (%) was calculated by using
the formula.
Percentage (%) of inhibition = [(absorbance of control–absorbance
of sample)/(absorbance of control)] ×100.
13](https://image.slidesharecdn.com/raheejc2-240425054113-4bca875a/75/Extraction-and-isolation-of-flavonoid-quercetin-13-2048.jpg)