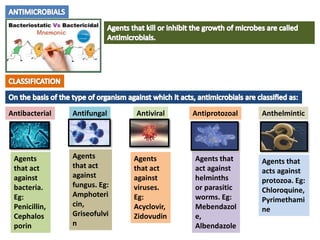
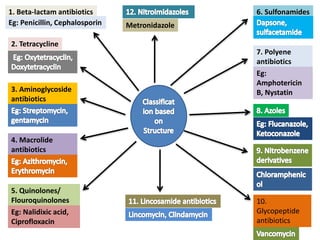
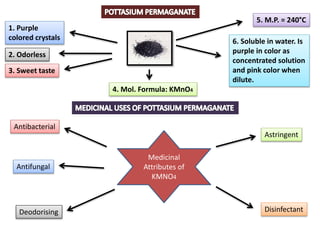
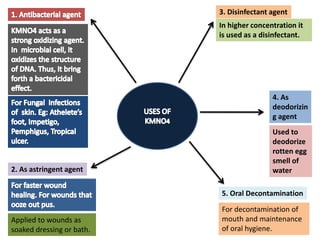
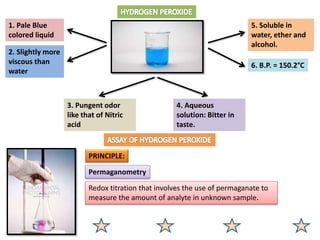
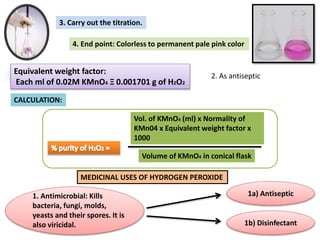
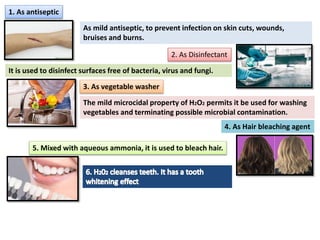
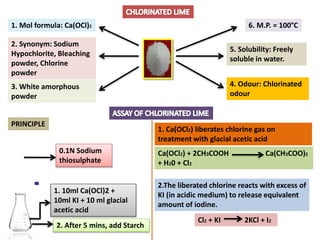
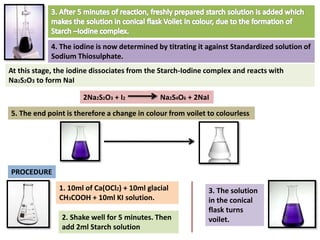
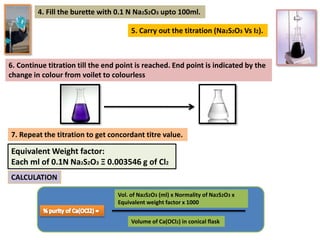
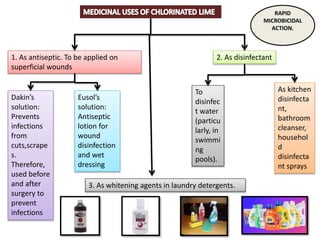
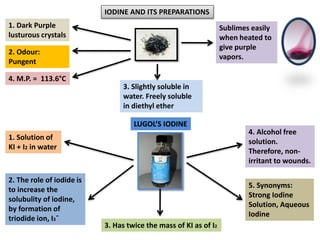
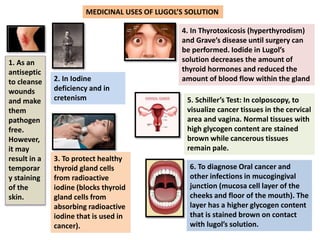
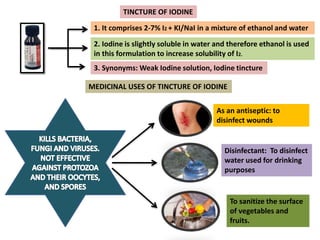
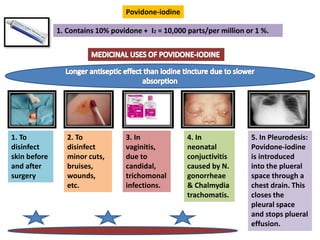
The document provides a comprehensive overview of various antimicrobial agents including antibacterial, antifungal, antiviral, antiprotozoal, and anthelmintic agents, along with examples and their medicinal uses. It discusses specific compounds such as potassium permanganate, hydrogen peroxide, and iodine solutions, detailing their properties, application methods, and chemical reactions involved in titrations and disinfectant properties. Additionally, it outlines the significance of these agents in clinical settings for treating infections and preventing microbial contamination.