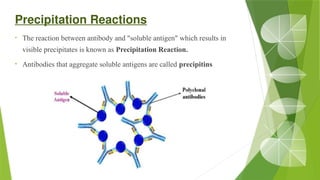
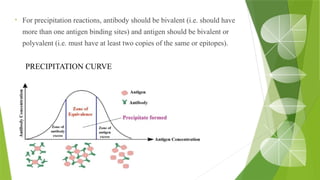
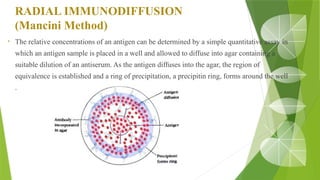
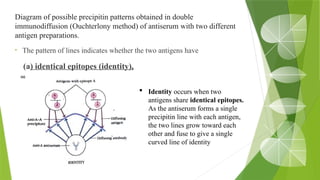
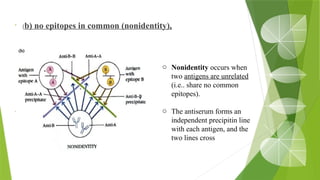
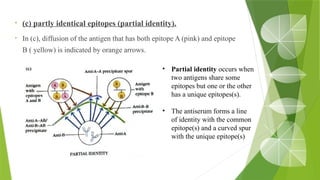
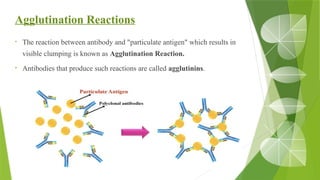
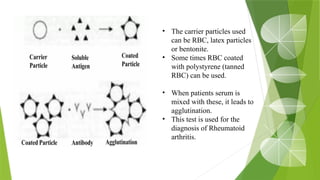
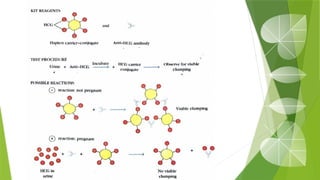
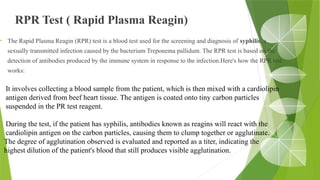
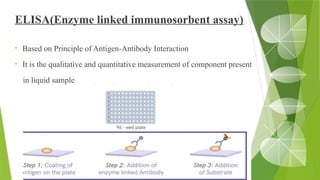
The document discusses the antigen-antibody interaction, a crucial process in immunology that enables the immune system to recognize and neutralize pathogens through highly specific binding mechanisms. It covers non-covalent interactions governing this binding, along with concepts such as antibody affinity, avidity, cross-reactivity, and the mechanisms of precipitation and agglutination reactions, which are essential for diagnostic assays in clinical settings. Various methods and tests, including the RPR test for syphilis and the ASO test for rheumatic fever, are highlighted for their applications in identifying antigens and antibodies.







![• Ka = affinity constant
• [Ab] = molar concentration of unoccupied binding sites on the antibody
• [Ag] = molar concentration of unoccupied binding sites on the antigen
• [Ab-Ag] = molar concentration of the antibody-antigen complex
Kd is a quantitative indicator of the stability of an Ag-Ab complex; very stable
complexes have very low values of Kd and less stable ones have higher values.
The association constant, K, can be determined by equilibrium dialysis or
by various newer methods.](https://image.slidesharecdn.com/antigenantibodyinteractioncopy-241123045606-a5bad15d/85/Antigen-Antibody-Interaction-9-320.jpg)