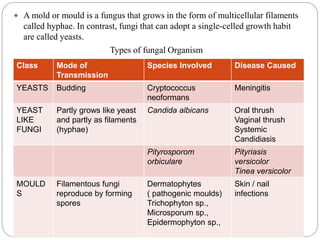
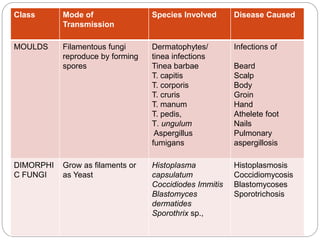
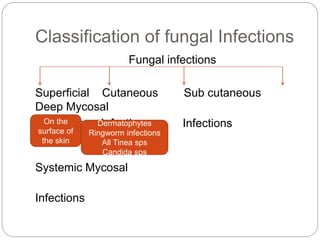
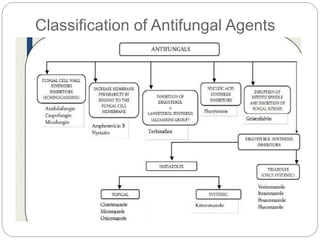
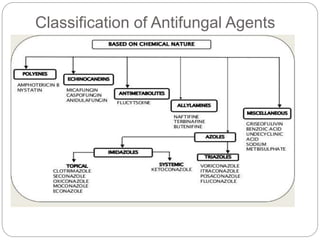
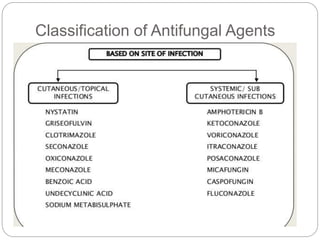
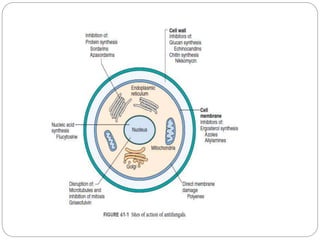
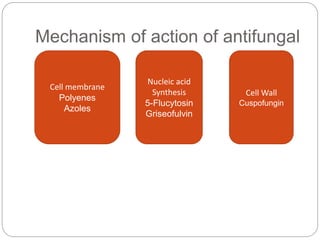
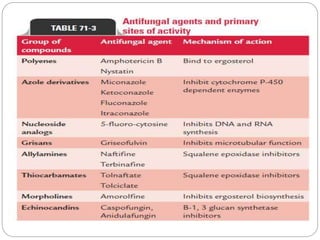
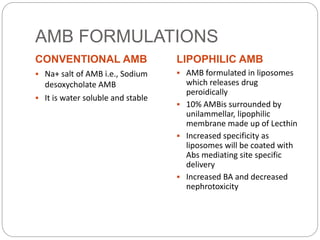
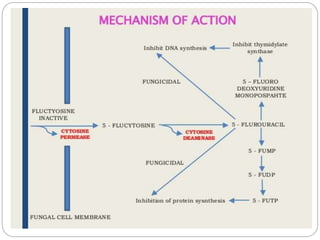
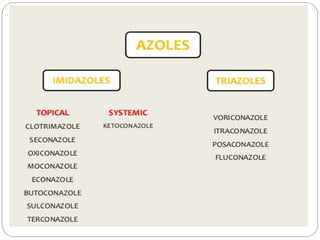
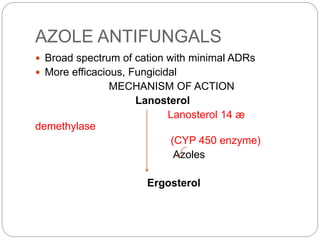
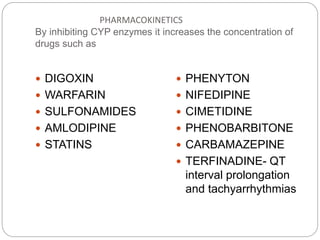
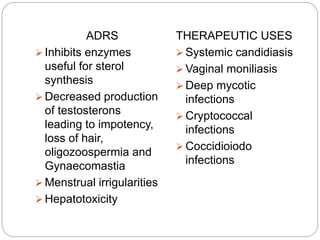
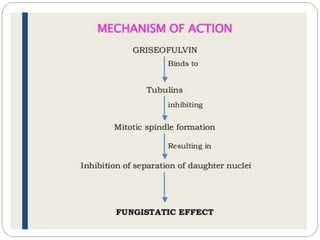
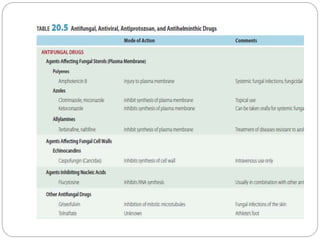
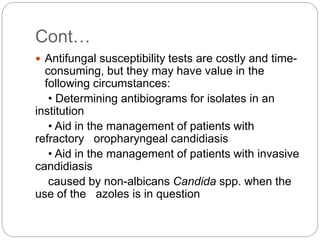
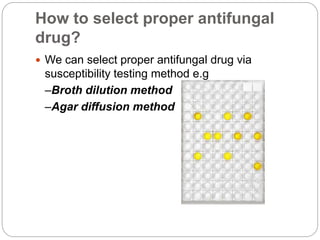
This document provides information about antifungal agents. It begins with an introduction to fungi and fungal infections. It then discusses the classification, mechanisms of action, and clinical uses of various antifungal drug classes, including azoles, polyenes, allylamines, and echinocandins. Side effects and resistance mechanisms are also covered. The document aims to outline the potential targets and modes of action of different antifungal agents used in clinical practice.