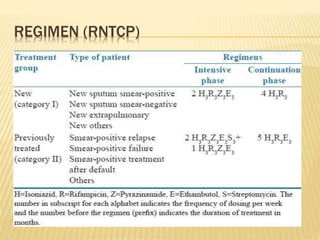
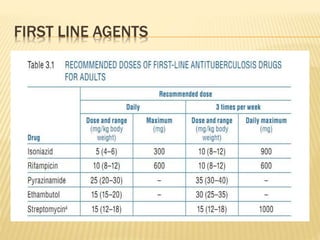
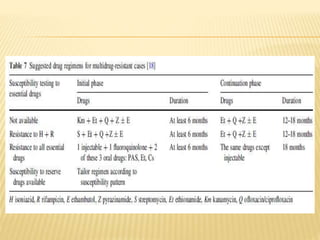
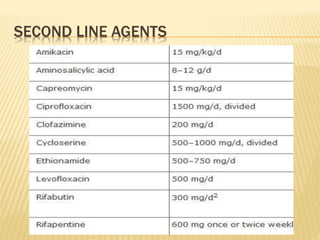
This document discusses anti-tuberculosis drugs used to treat bone tuberculosis. It describes the standard Category I treatment regimen of 2 months of isoniazid, rifampin, pyrazinamide, and ethambutol, followed by 4 months of isoniazid and rifampin. While treatment duration was historically long, modern drugs achieve high bone concentrations and shorter regimens are effective. The document also details first-line drugs like isoniazid and rifampin and second-line drugs used to treat multidrug-resistant tuberculosis. Adverse effects and mechanisms of action are provided for each major anti-tuberculosis medication.