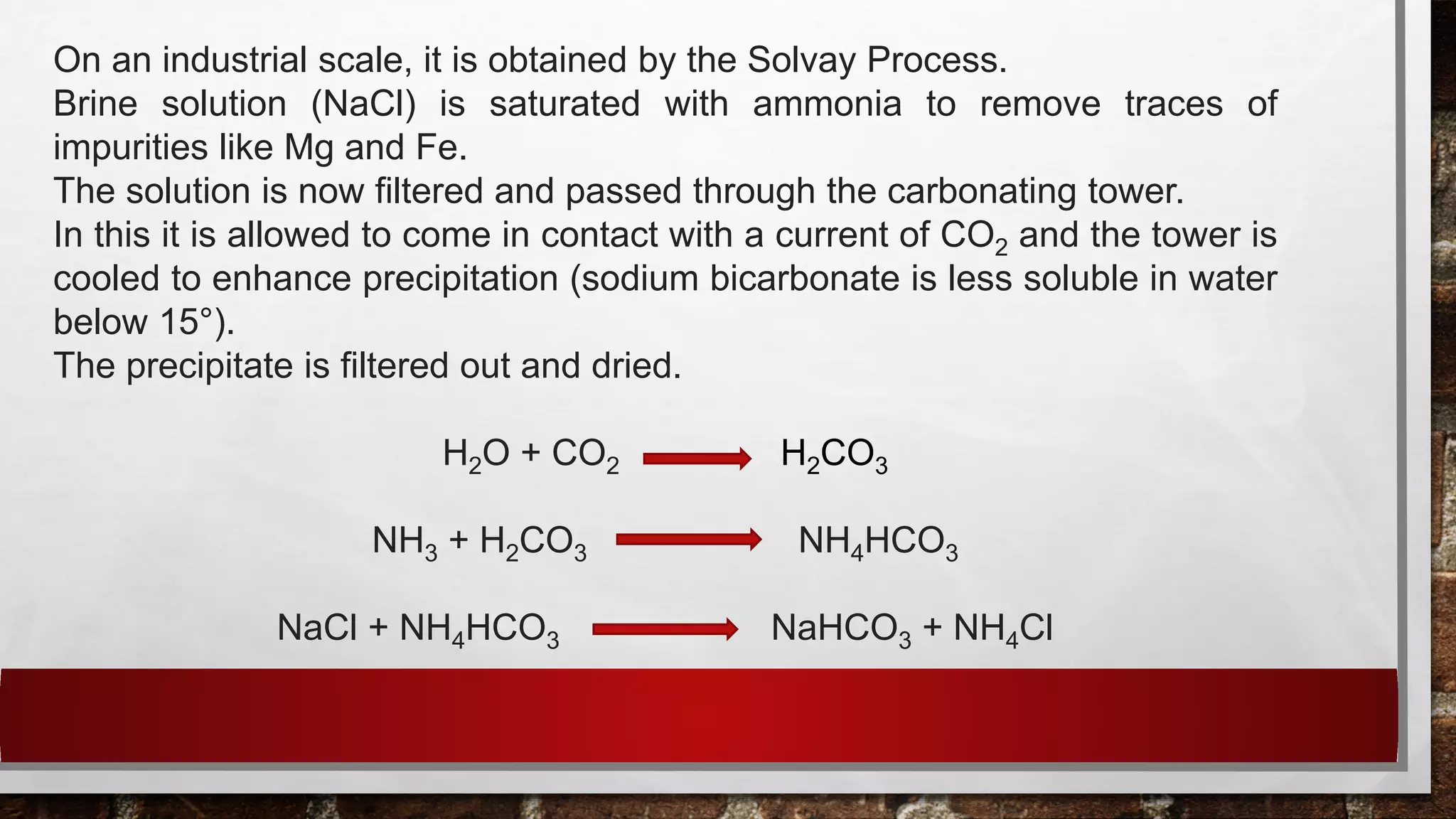
The document discusses antacids and their ideal requirements. It states that no single antacid satisfies all requirements, so combinations are used. It then lists some common combination antacid preparations, including their ingredients. It also provides information about the properties, preparation and uses of some individual antacids, including sodium bicarbonate, aluminium hydroxide gel, and magnesium hydroxide.