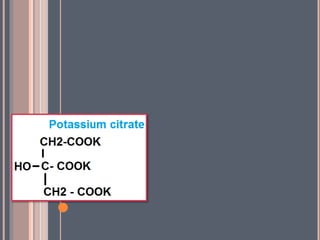
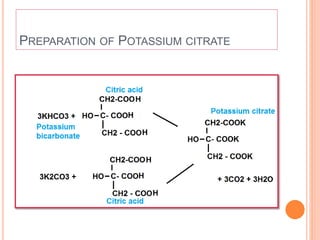
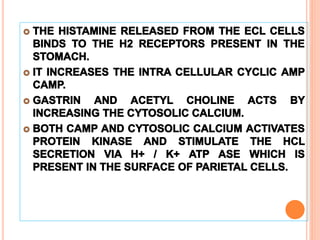
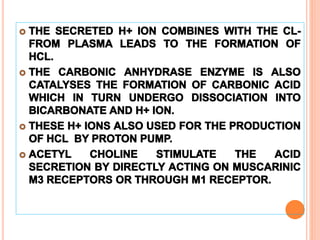
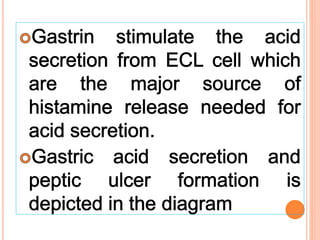
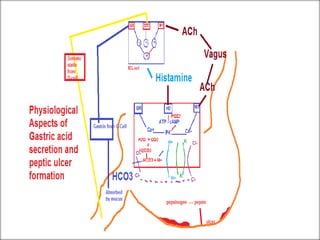
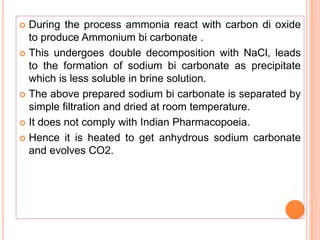
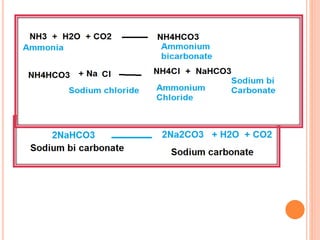
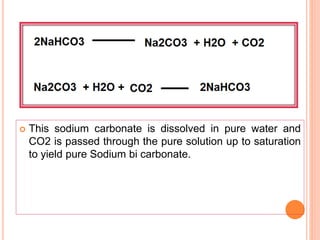
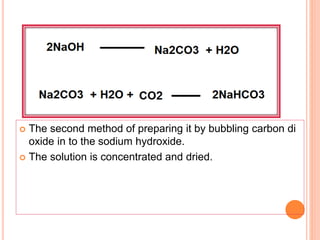
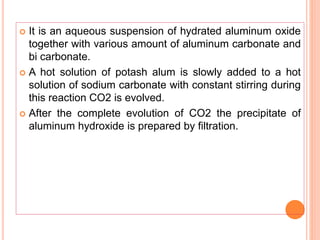
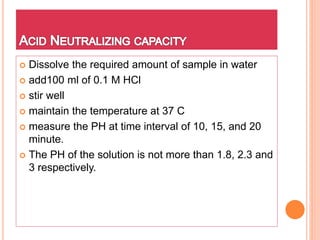
The document discusses various types of antacids, their formulations, mechanisms of action, and precautions. It describes that antacids work by neutralizing stomach acid but their effects only last 20-40 minutes when taken without food. Several antacid brands and their active ingredients are outlined, including Gelusil, Gelusil MPS, and Digene. Potential side effects of antacids like acid rebound, milk-alkali syndrome, and interactions with other drugs are also noted. Manufacturing processes for ingredients like sodium bicarbonate, aluminum hydroxide gel, and potassium citrate are summarized as well.








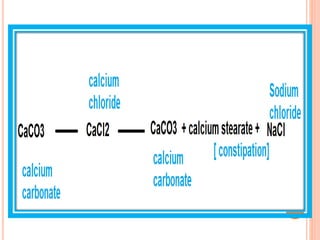




































![ Ammonia soda process or solvay process
Introduced by Brunner and Mond
Strong brine [ Brine solution – high concentration
of Nacl ]
solution passed through carbonating water
saturated with Ammonia
It is again saturated with carbon di oxide under
pressure](https://image.slidesharecdn.com/antacid-180217080837/85/Antacid-57-320.jpg)
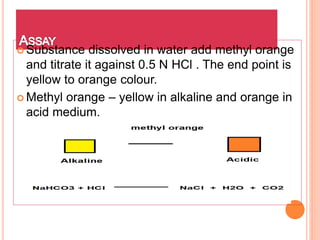





![ Substance dissolved in HCl by warming in water
bath to this excess amount of EDTA is added. The
solution is then neutralised with NaOH using methyl
red as indicator.[ The color change from red to
yellow.] To this solution hexamine is added as buffer
and xylenol orange as an indicator. Titrate it against
lead nitrate.](https://image.slidesharecdn.com/antacid-180217080837/85/Antacid-70-320.jpg)