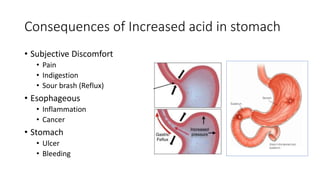
This document discusses antacids, which are basic substances that neutralize gastric acid and raise the pH of gastric contents. It describes the normal physiology of gastric acid secretion and factors that can increase secretion. Increased acid can lead to discomfort, inflammation, ulcers, and cancer. Antacids are classified as systemic or non-systemic. Systemic antacids like sodium bicarbonate are absorbed but can cause alkalosis, while non-systemic antacids like magnesium hydroxide, aluminum hydroxide, and calcium carbonate react locally in the stomach. Combination antacids contain fast- and slow-acting components to provide prompt and sustained relief without systemic effects or disturbing bowel movements.