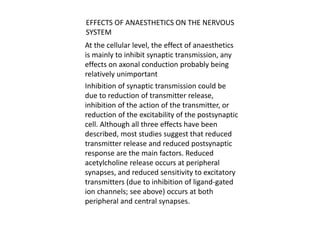
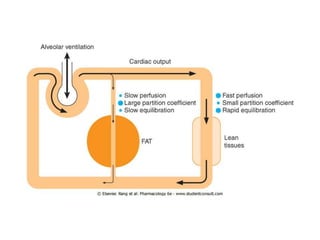
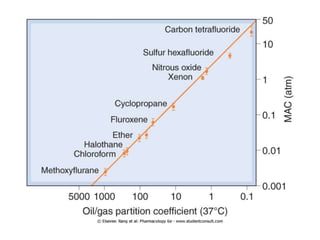
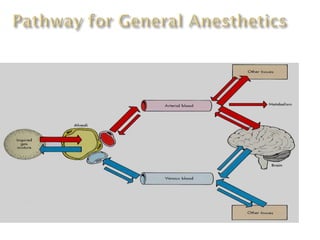
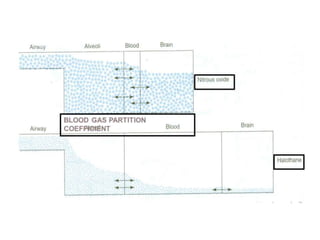
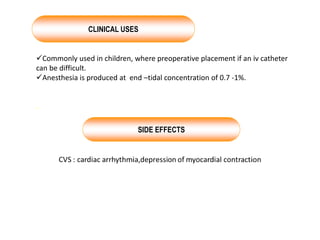
General anesthetics are drugs that produce reversible loss of sensation and consciousness. They work by depressing brain function from the cortex down to the medulla. There are two main types - inhalational anesthetics which are gases or vapors inhaled, and intravenous anesthetics which are injected. Both work by inhibiting excitatory neurotransmitters like glutamate while enhancing inhibitory neurotransmitters like GABA. This raises the threshold for electrical signals in the brain and lowers responsiveness to stimuli. While very effective at inducing unconsciousness, general anesthetics must be carefully monitored as the line between surgical anesthesia and respiratory/circulatory failure is narrow.

![DEFINITION
GENERAL ANESTHETICS [GAs] ARE DRUGS
WHICH PRODUCE REVERSIBLE LOSS OF ALL
SENSATION AND CONSCIOUSNESS SO IT CAN BE
DIFFERENTIATED FROM SLEEP,HEAD
INJURY,HYPNOSIS,DRUG POISONING AND COMA.](https://image.slidesharecdn.com/anesthesia-170313131536/85/Anesthesia-3-320.jpg)








![(a).INHALATIONAL ANAESTHESIA
- Inhalational anesthesia is achieved through
airway tract by facemask, laryngeal mask or
endotracheal tube.
- The agent used is a gas like nitrous oxide or
volatile vapor like chloroform, ether, or
flothane.
- Inhalational anesthesia depresses the brain
from up [cortex] to down [the medulla] by
increasing dose.](https://image.slidesharecdn.com/anesthesia-170313131536/85/Anesthesia-12-320.jpg)