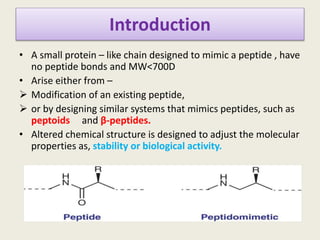
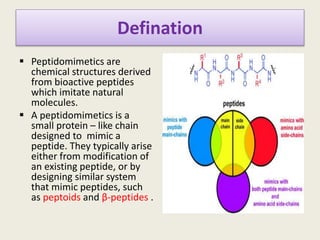
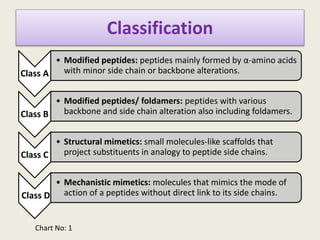
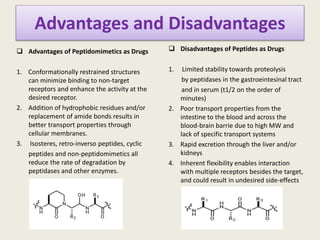
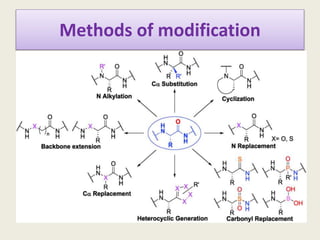
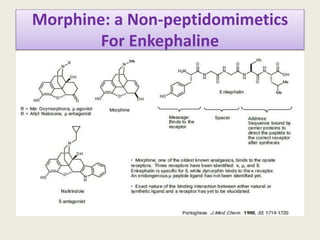
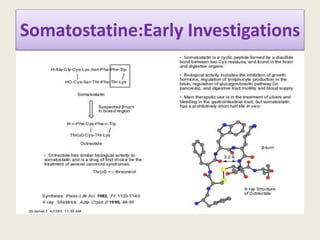
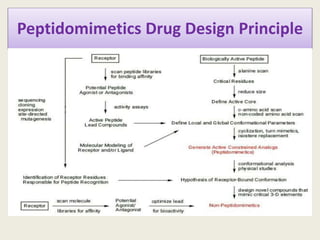
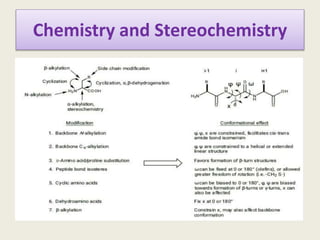
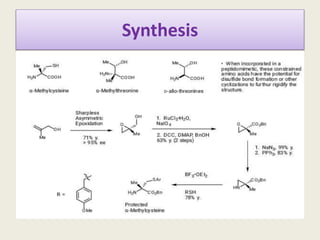
Peptidomimetics are small protein-like chains designed to mimic peptides and can be derived from existing peptides or designed structures, aiming to improve stability and biological activity. The document discusses their classification, advantages such as enhanced activity and better transport properties, and disadvantages like limited stability and potential side effects. Additionally, it covers methods of modification and highlights recent advancements, including their application in peptide vaccines and antimicrobial peptides.



![Therapeutic Value of Peptidomimetics
INNs Brand names Length Sequences Companies Indications Half-life
Daptomycin Cubicin 13 aa N-Decanoyl-Trp-Asp-Asp-
threonylglycyl-ornithyl-Asp-D-
alanyl- aspartylglycyl-D-seryl-
threo-3-methyl- glutamyl-3-
anthraniloyl-alanine[egr]1-
lactone
Cubist
Pharmaceutical
s
Antibiotic used to treat
systemic and life-
threatening infections
caused by Gram-
positive organisms
8–9
hours
Afamelanotid
e
Scenesse 13 aa Ac-Ser-Tyr-Ser-Nle-Glu-His-D-
Phe-Arg- Trp-Gly-Lys-Pro-Val-
NH2
Clinuvel
Pharmaceutical
s
Erythropoietic
porphyries
100 min
Eptifibatide Integrilin 7 aa c[Mpa-homoArg-Gly-Asp-Trp-
Pro-Cys]- NH2
Millennium
Pharms, GSK,
Schering-Plough
Acute coronary
syndrome, unstable
angina undergoing PCI
2.5 hours
Cyclosporine Neoral, Gengraf,
Sandimmune,
Arpimune Me,
Graftin,
Imusporin,
Panimun Bioral
11 aa (3S,6S,9S,12R,15S,18S,21S,24S,
30S,33S)- 30-Ethyl-33-
[(1R,2R,4E)-1-hydroxy-2-
methyl-4-hexen-1-yl]-
6,9,18,24- tetraisobutyl-3,21-
diisopropyl-
1,4,7,10,12,15,19,25,28-
nonamethyl-
1,4,7,10,13,16,19,22,25,28,31-
undecaazacyclotritriacontane-
2,5,8,11,14,17,20,23,26,29,32-
undecone
RGP life
sciences,
Ranbaxy
laboratories,
Cipla limited,
Ecolity, Allergan,
Novartis
Transplant (kidney,
liver, and heart)
rejection, rheumatoid
psoriasis arthritis,
severe
5–18
hours](https://image.slidesharecdn.com/53-191219102328/85/PEPTIDOMIMETICS-M-PHARM-BSC-MSC-B-PHARM-15-320.jpg)

![PEPTIDOMIMETICS [M.PHARM, BSC, MSC, B.PHARM]](https://image.slidesharecdn.com/53-191219102328/85/PEPTIDOMIMETICS-M-PHARM-BSC-MSC-B-PHARM-17-320.jpg)