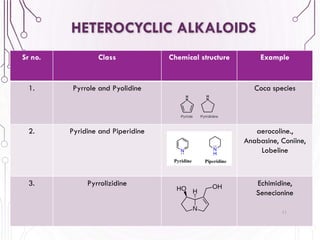
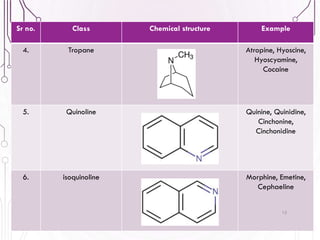
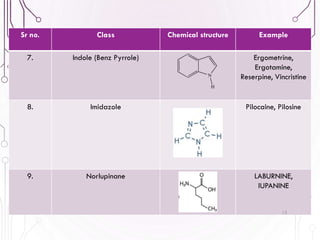
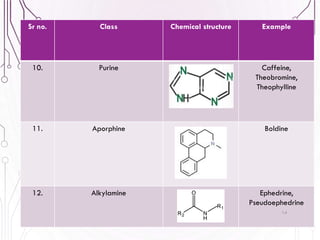
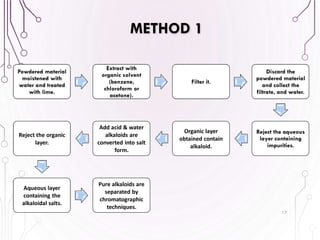
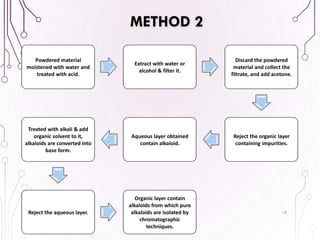
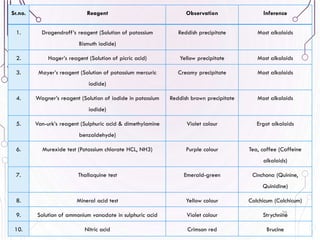
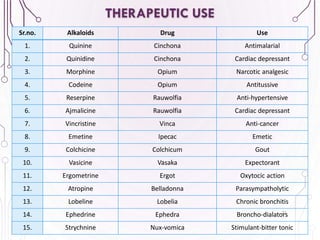
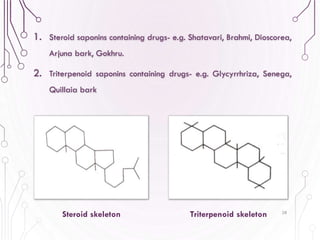
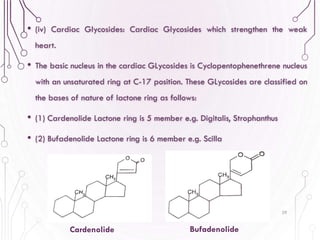
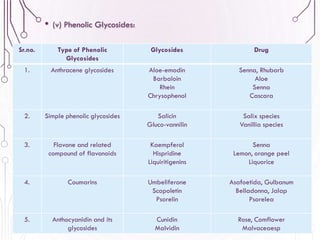
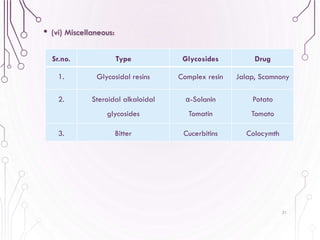
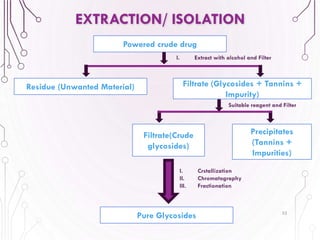
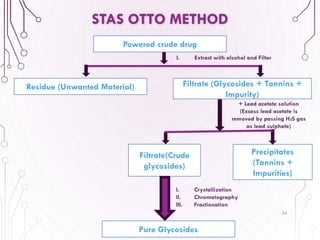
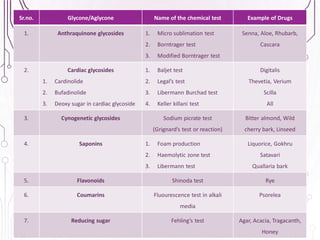
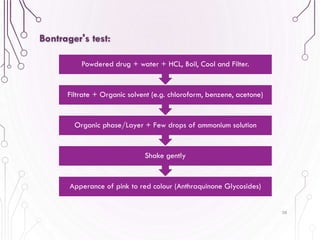
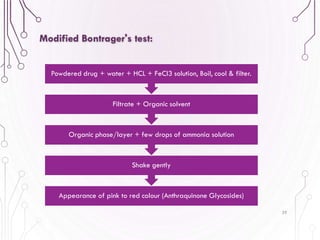
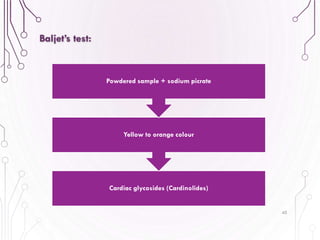
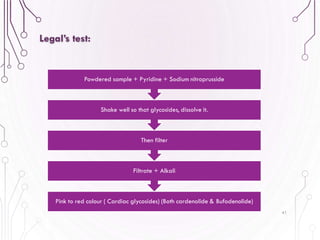
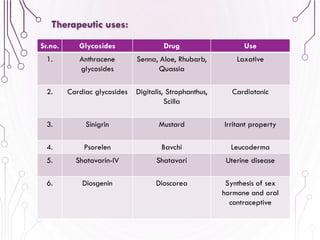
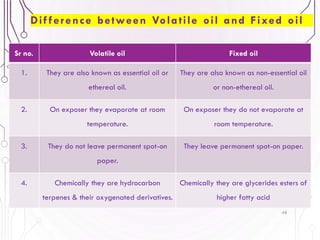
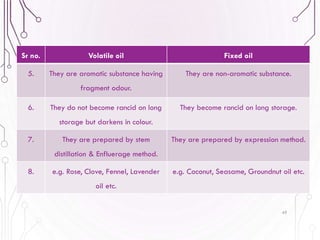
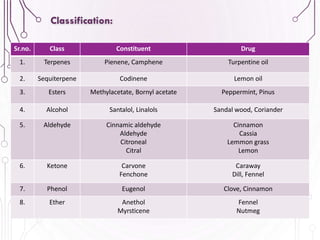
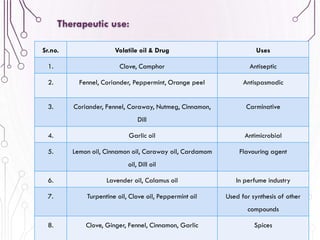
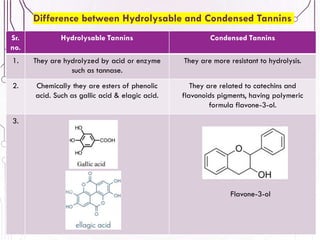
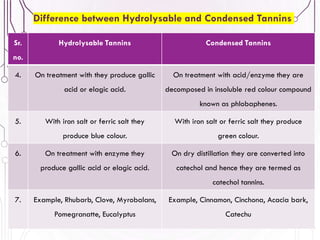
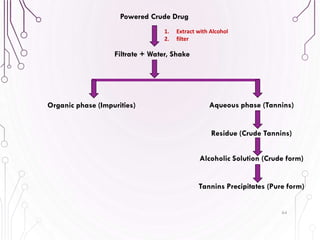
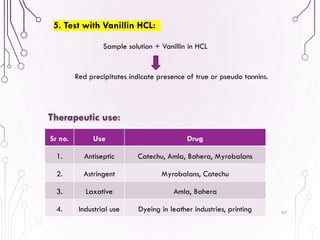
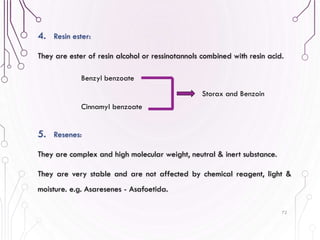
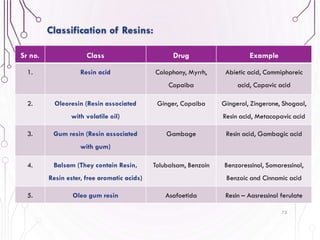
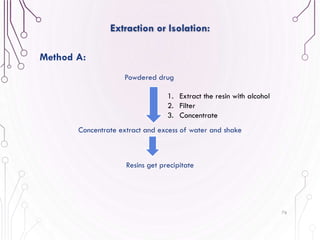
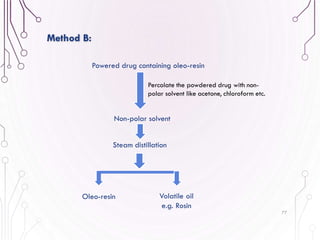
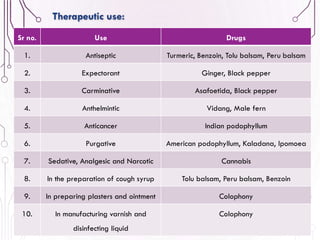
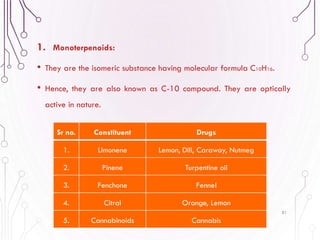
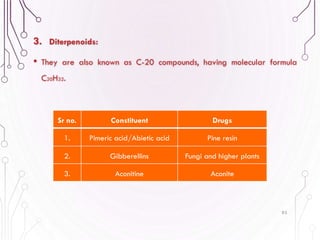
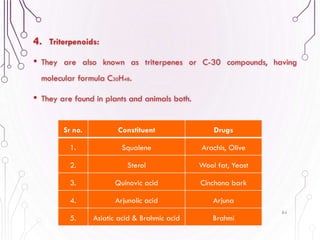
The document provides a comprehensive overview of various phytochemical compounds including alkaloids, terpenoids, glycosides, volatile oils, tannins, and resins, focusing on their occurrence, properties, classifications, extraction, identification tests, and therapeutic applications. Alkaloids are detailed in terms of their chemical structure, solubility, and pharmacological uses, while glycosides are discussed regarding their hydrolysis, classification, and extraction methods. Additionally, the document outlines various identification tests for both alkaloids and glycosides, listing specific reagents and their corresponding reactions.