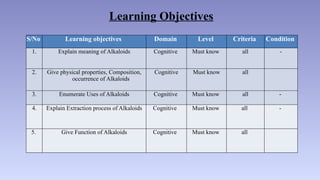
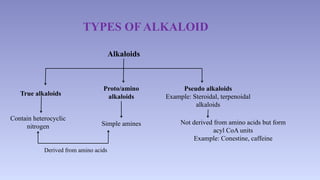
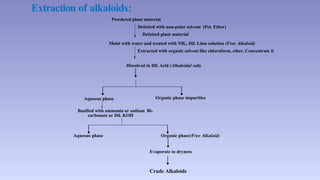
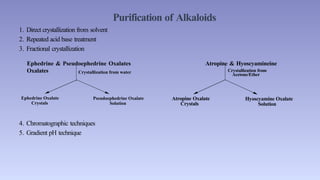
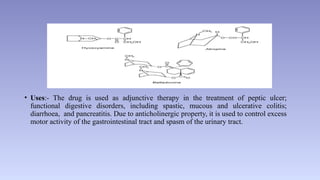
The document discusses alkaloids, a group of naturally occurring chemical compounds, detailing their identification, properties, types, distribution in plants, and extraction processes. It highlights their pharmacological activities and therapeutic applications, including uses in treating serious medical conditions. The document also provides information on the classification of alkaloids and notable examples such as belladonna and ephedra.