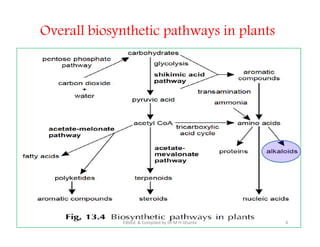
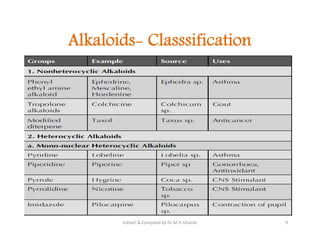
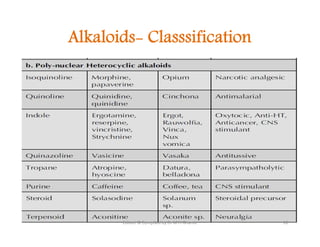
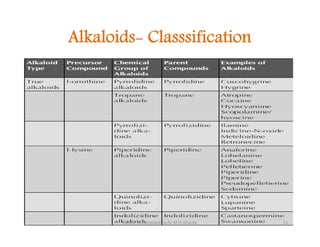
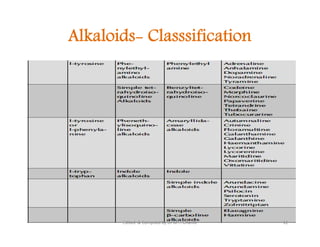
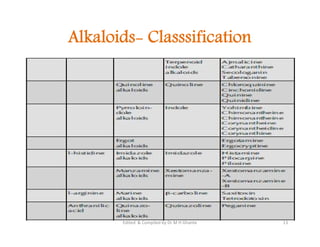
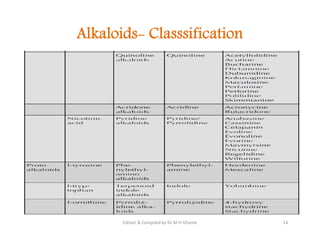
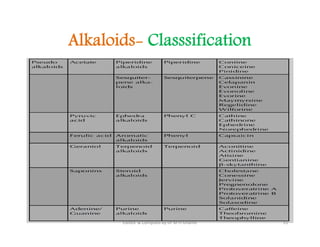
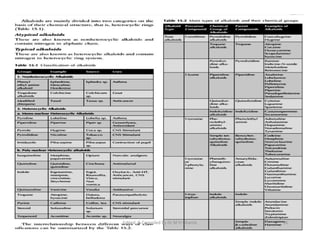
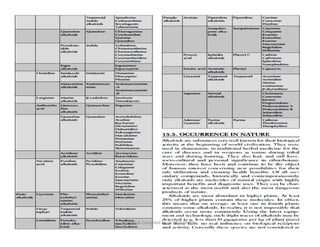
The document discusses various topics related to pharmacognosy and phytochemistry, including different classes of plant-derived compounds and specific examples. It begins with a general introduction and overview of alkaloids, describing their properties, classification, extraction methods, and important historical discoveries. Specific alkaloids discussed include those found in belladonna, such as hyoscyamine, atropine, and apoatropine. The chemical constituents and tests used to identify alkaloids are also summarized.