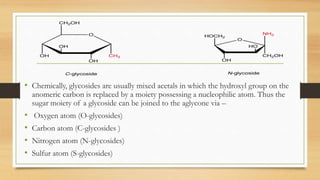
Glycosides are compounds that contain a sugar component bonded to another moiety. They are classified based on the type of sugar, nature of the aglycone, and type of glycosidic linkage. Glycosides have various medicinal uses including as cardiac drugs, laxatives, analgesics, and anti-inflammatory agents. They are isolated using extraction and purification techniques to obtain the pure glycoside component.