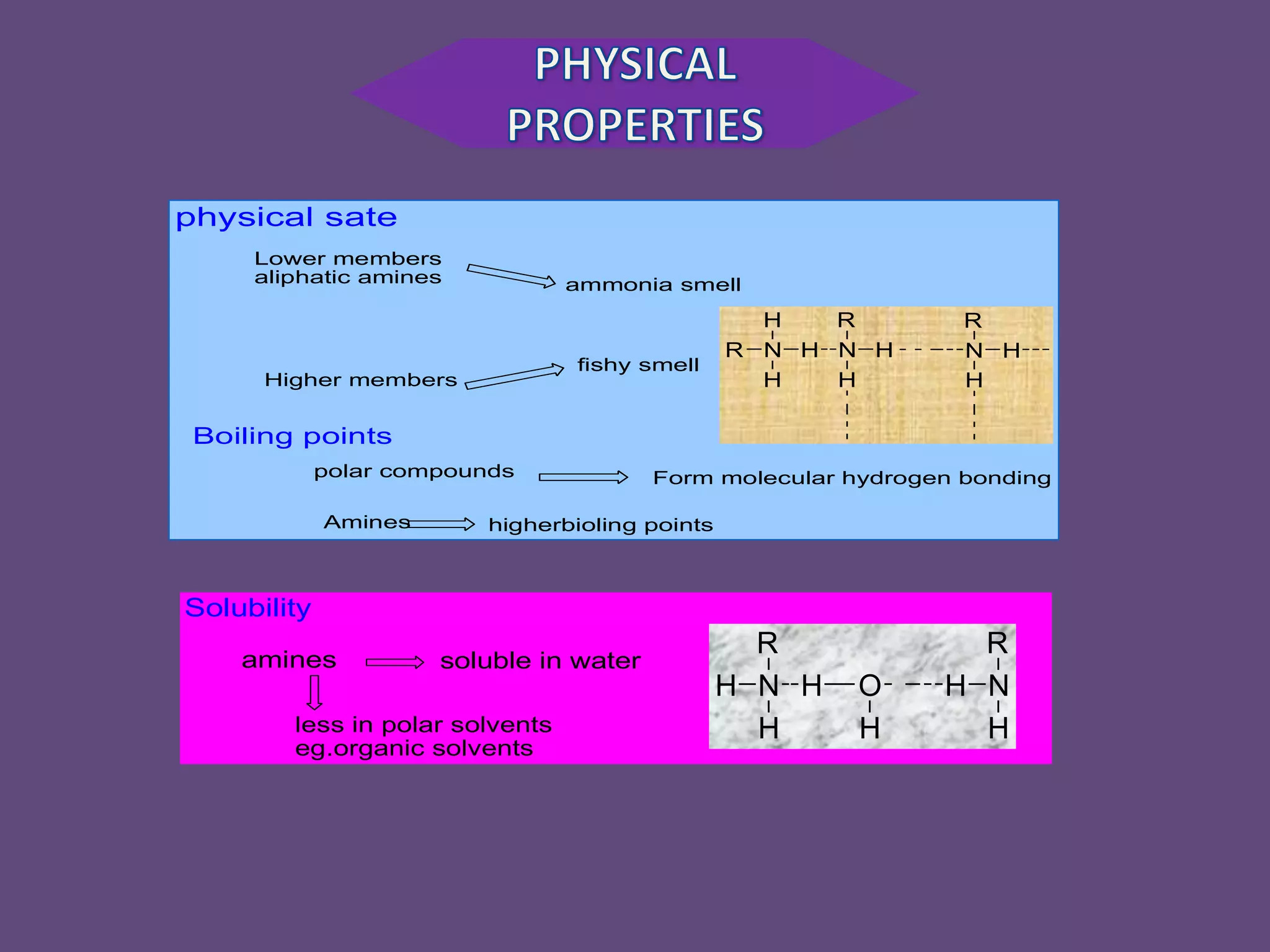
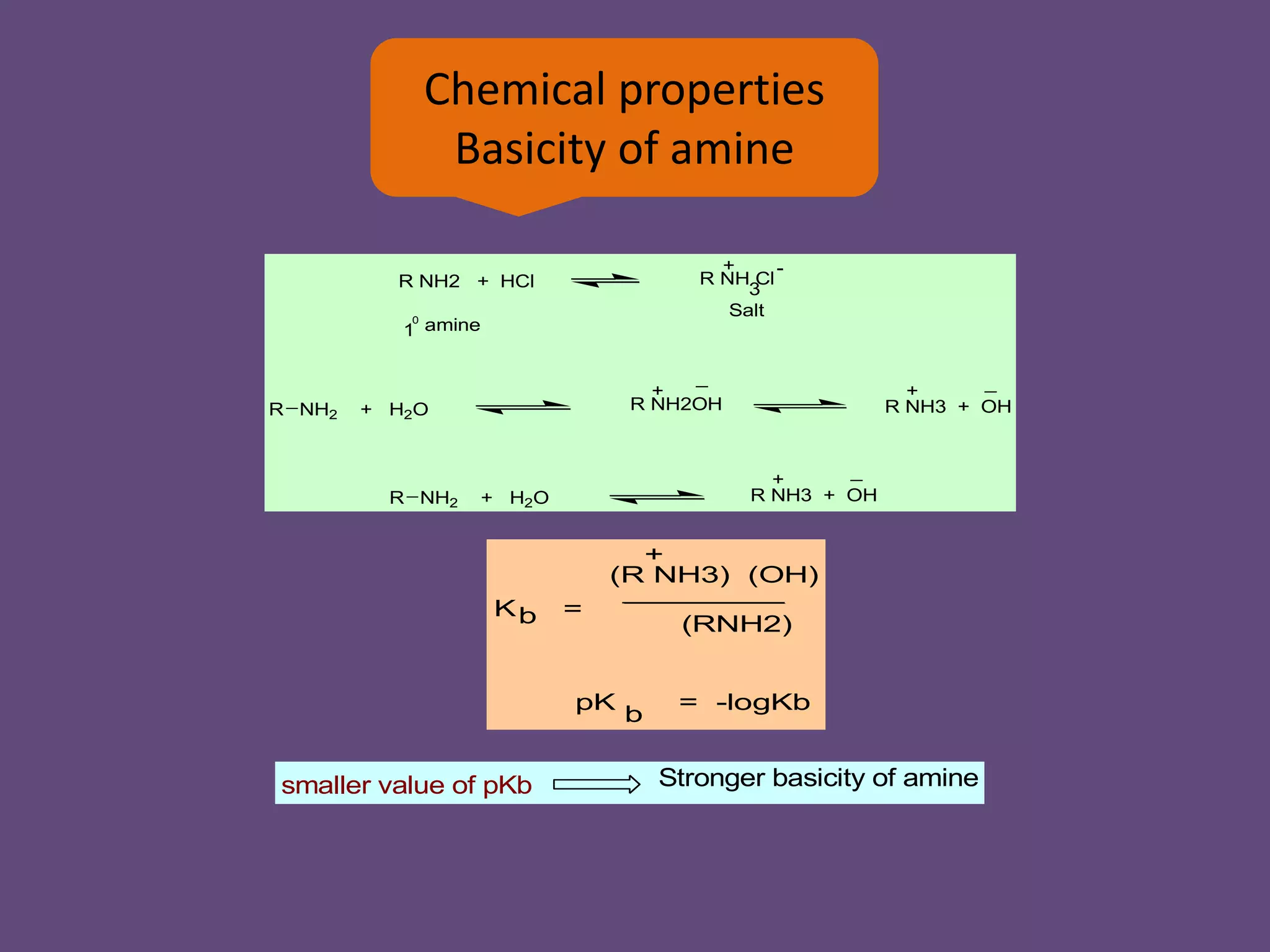
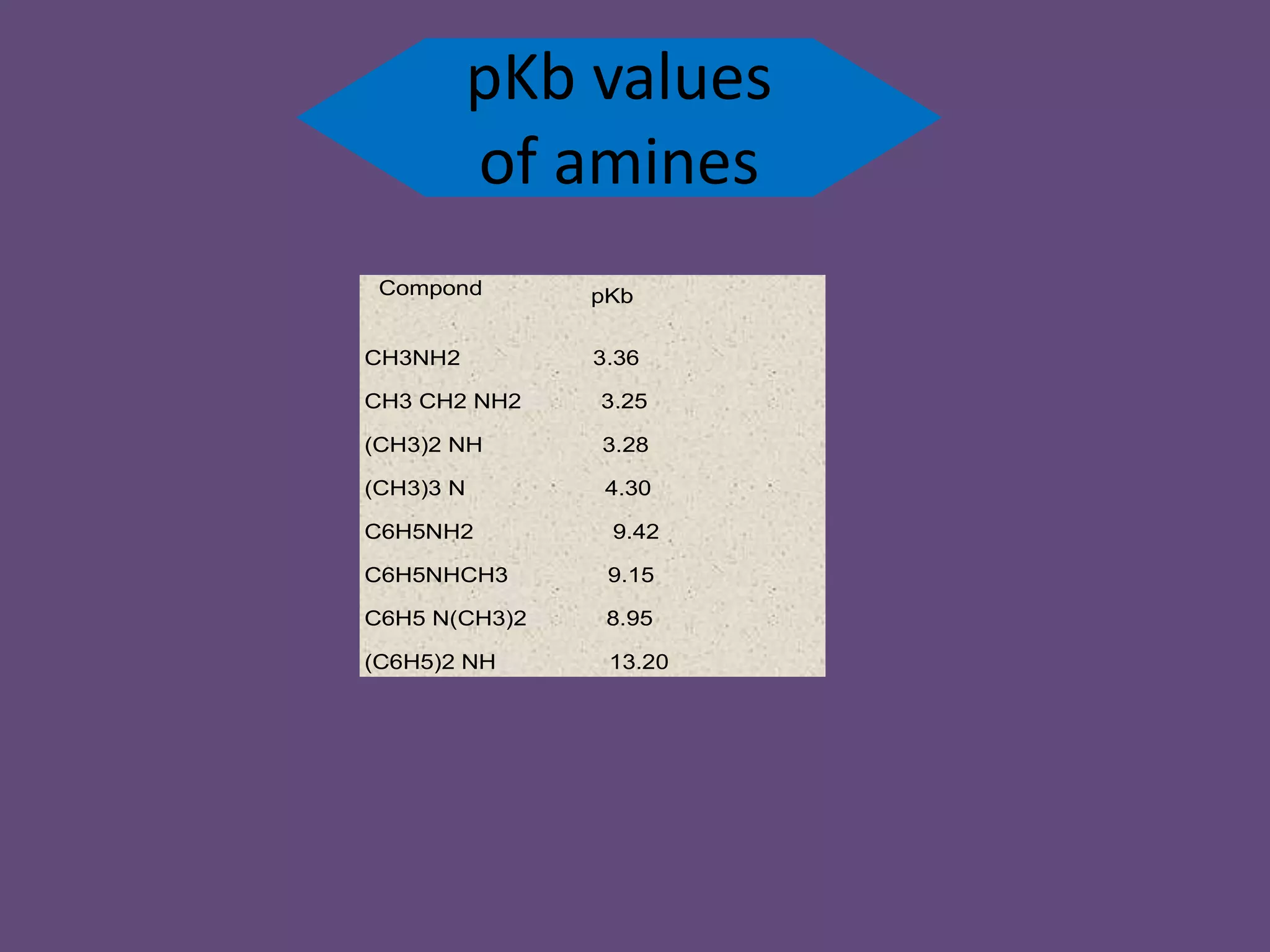
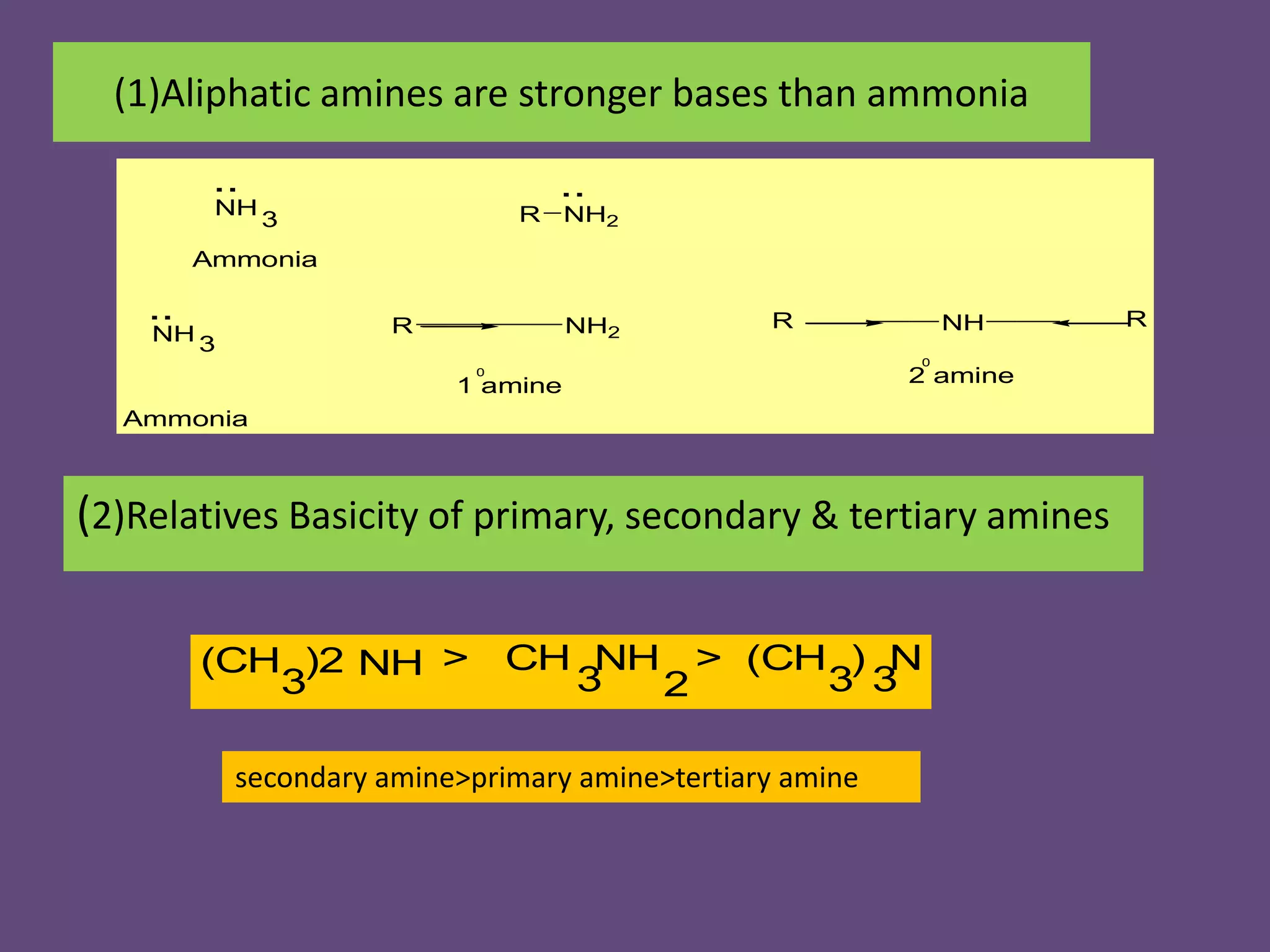
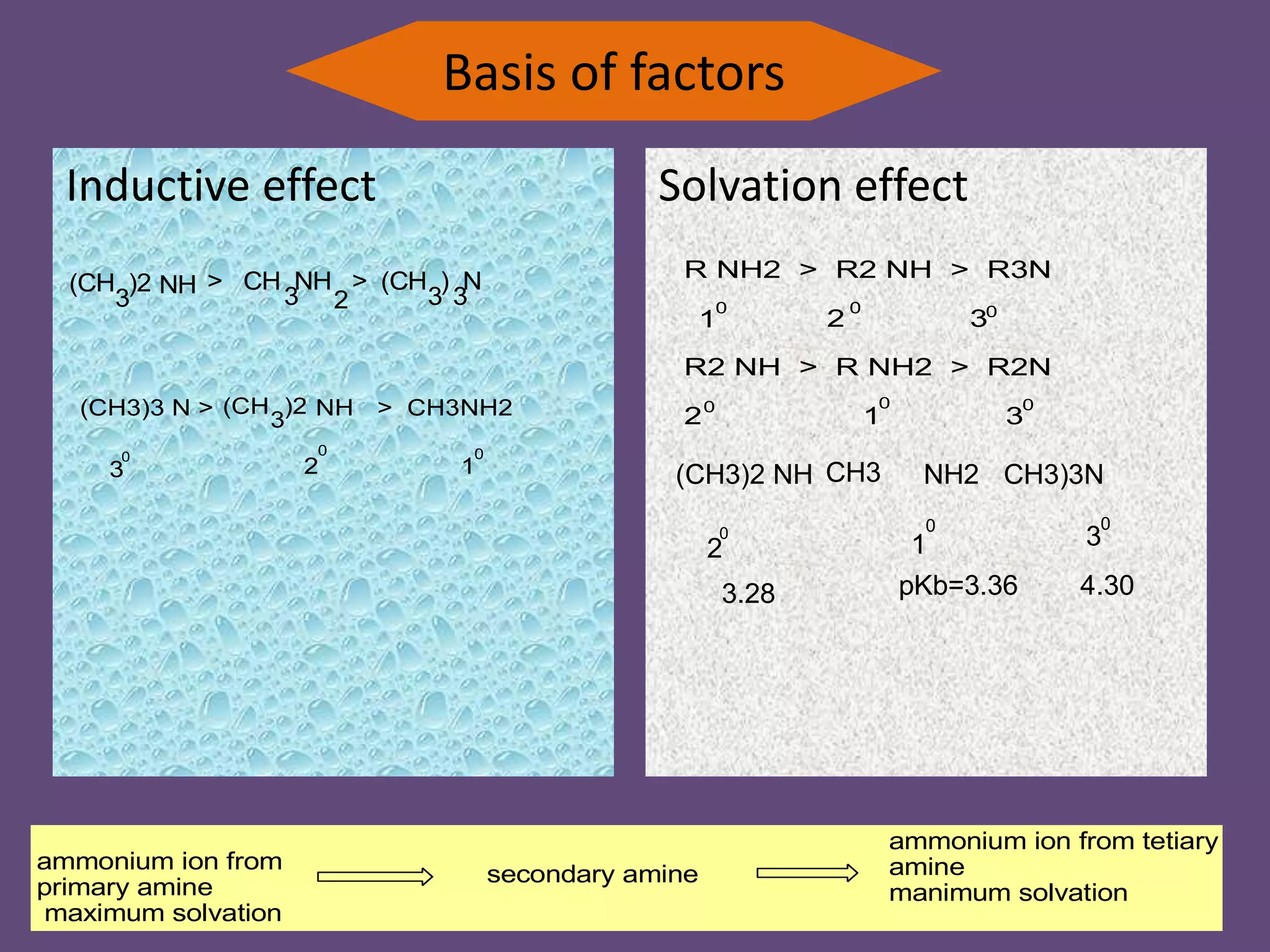
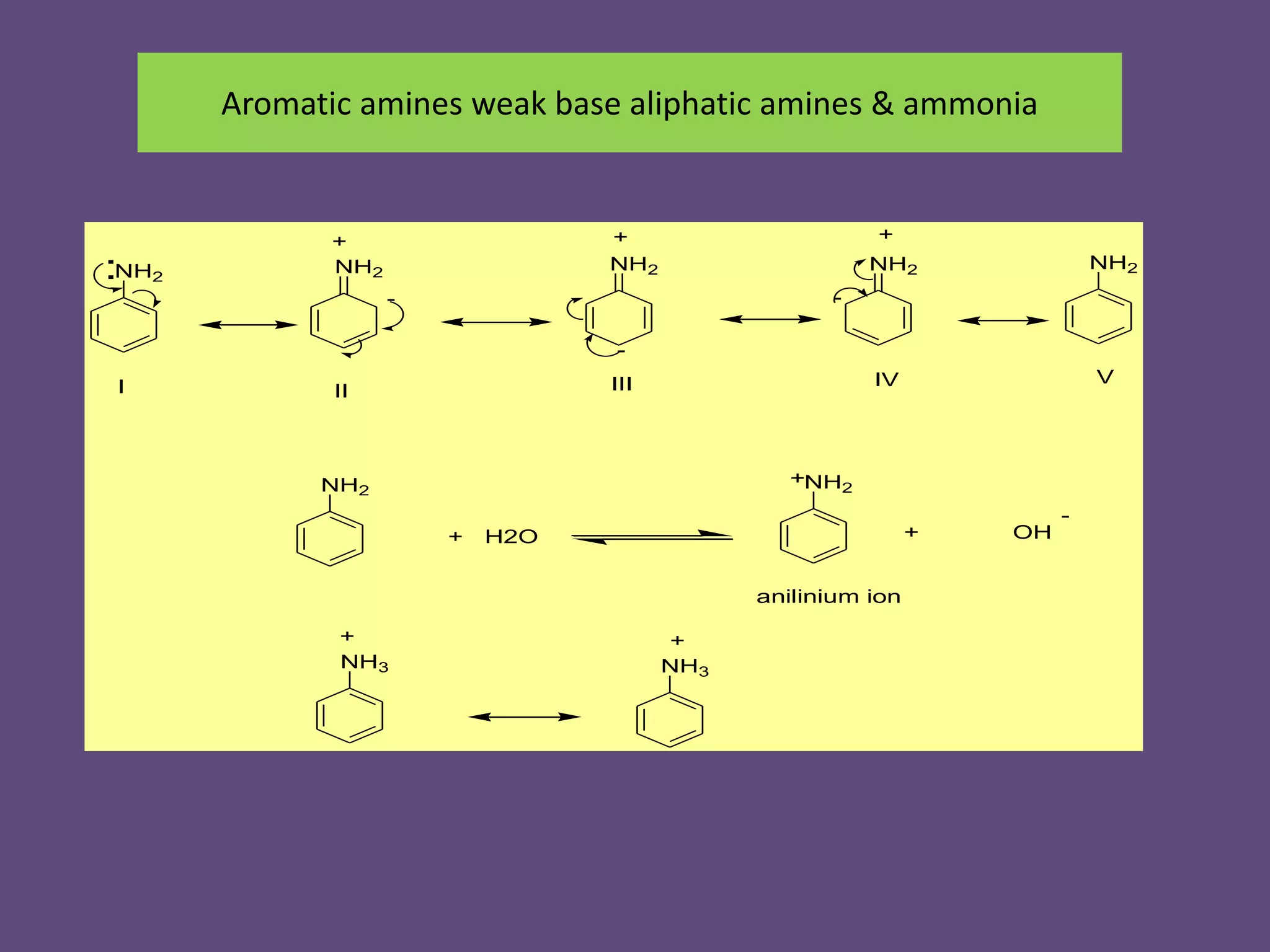
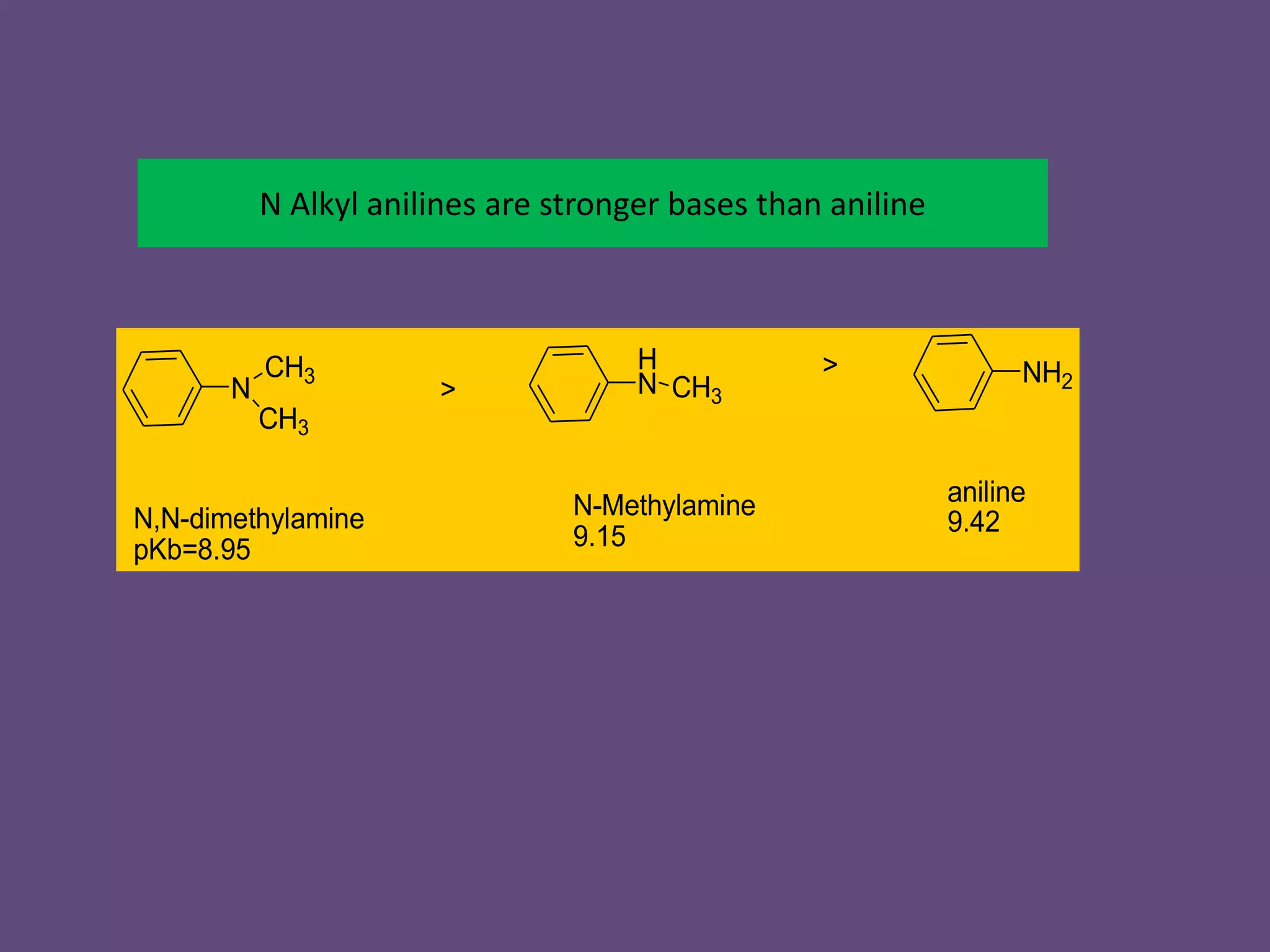
This document discusses the properties and chemical behavior of aliphatic amines, highlighting their basicity compared to ammonia and the influence of structural variations on basicity levels. It provides specific pKb values for various amines and compares their solubility and solvent interactions. The document concludes that primary amines are generally stronger bases than secondary and tertiary amines and outlines factors affecting amine basicity.