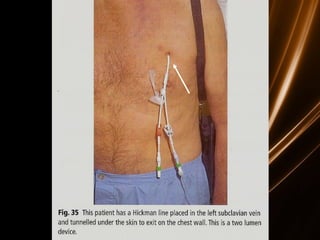
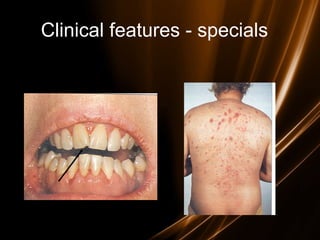
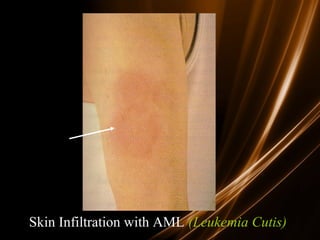
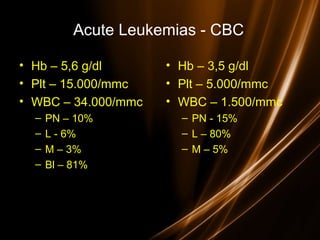
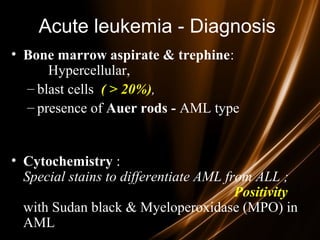
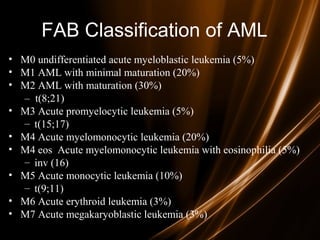
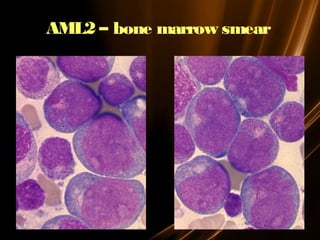
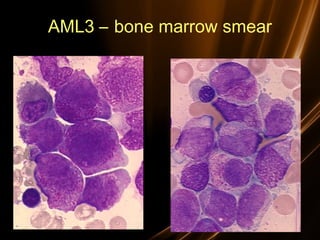
Acute leukemias are malignant disorders of hematopoietic tissues characterized by increased white blood cells in the bone marrow and blood. They are classified as either acute lymphoblastic leukemia (ALL) or acute myeloid leukemia (AML) based on the affected cell lineage. Treatment involves chemotherapy to induce remission through combination regimens, with the goals of restoring normal hematopoiesis and preventing relapse through additional consolidation therapy and long-term maintenance treatment. Prognosis depends on several risk factors like age, subtype, initial response to treatment, and specific genetic abnormalities.














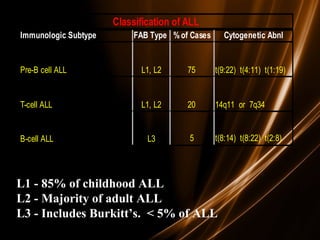
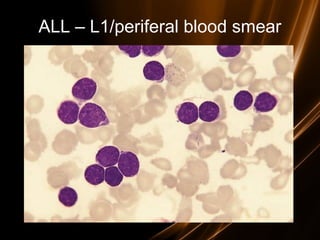
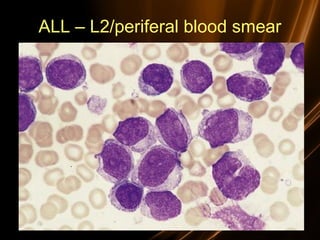
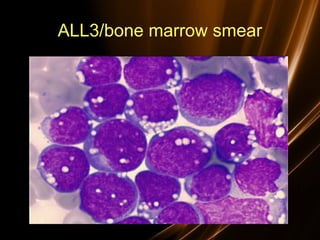


![Prognosis in ALL
• Good risk includes
– (1) no adverse cytogenetics,
– (2) age younger than 30 years
– (3) WBC count of less than 30,000/mL, and
– (4) complete remission within 4 weeks.
• Intermediate risk
– does not meet the criteria for either good risk or poor risk.
• Poor risk includes
– (1) adverse cytogenetics [(t9;22), (4;11)],
– (2) age older than 60 years,
– (3) precursor B-cell WBCs with WBC count greater than
100,000/mL, or
– (4) failure to achieve complete remission within 4 weeks.](https://image.slidesharecdn.com/hematologyneoplasia-181006161923/85/ACUTE-LEUKEMIA-41-320.jpg)