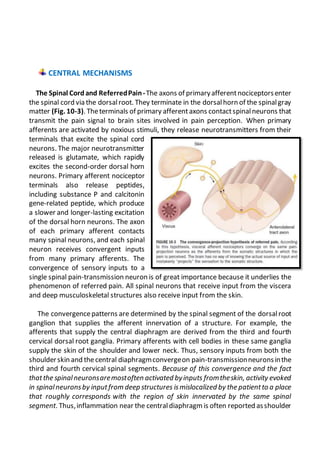
This document summarizes the pathophysiology of pain. It describes how pain is detected by nociceptors in the periphery and transmitted through the spinal cord and brain. Pain serves an important protective function but can also become chronic through peripheral and central sensitization. Psychological factors and brain circuits can also modulate pain perception. Damage to the peripheral or central nervous system can cause neuropathic pain, which is often severe and resistant to treatment.