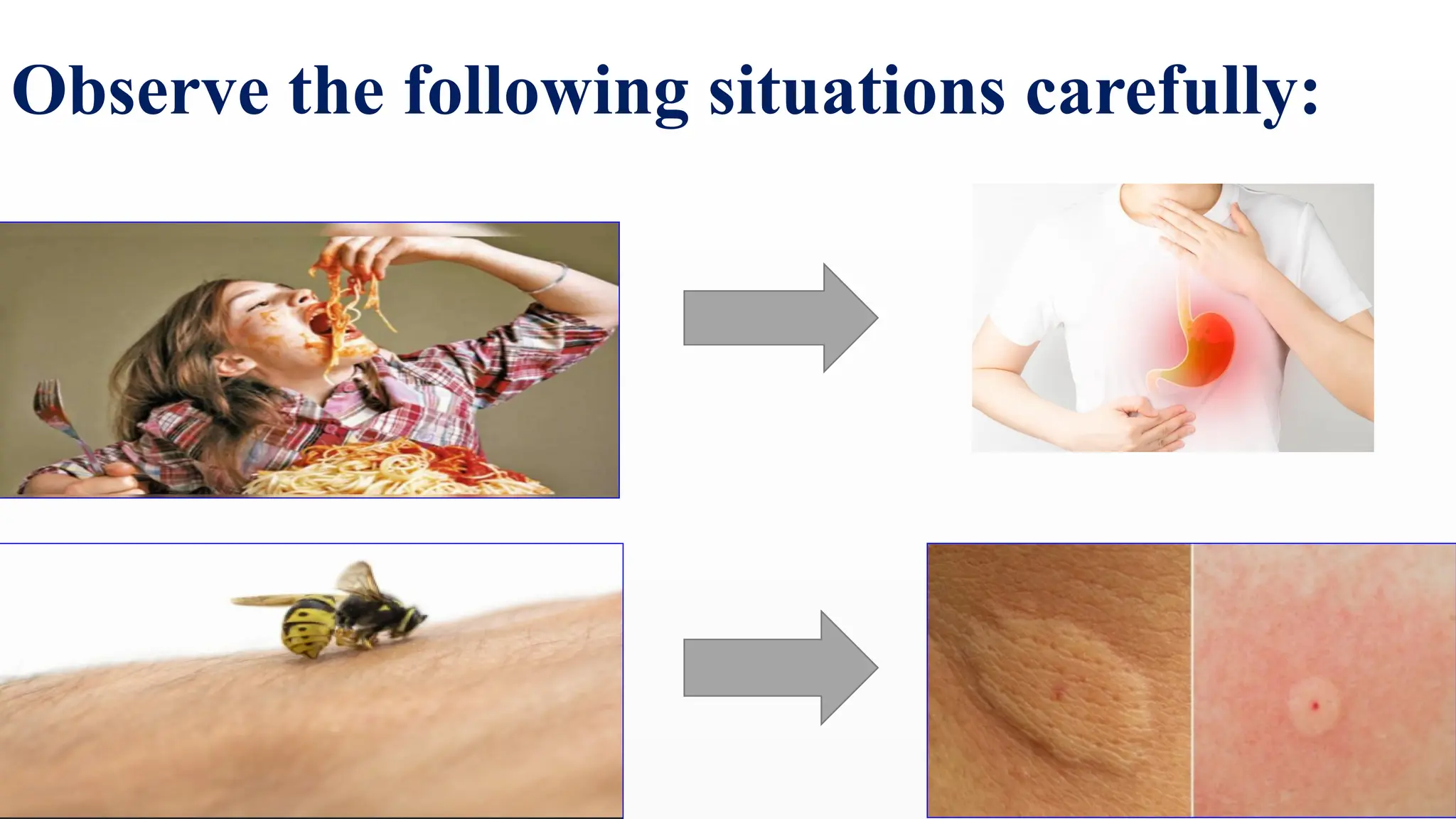
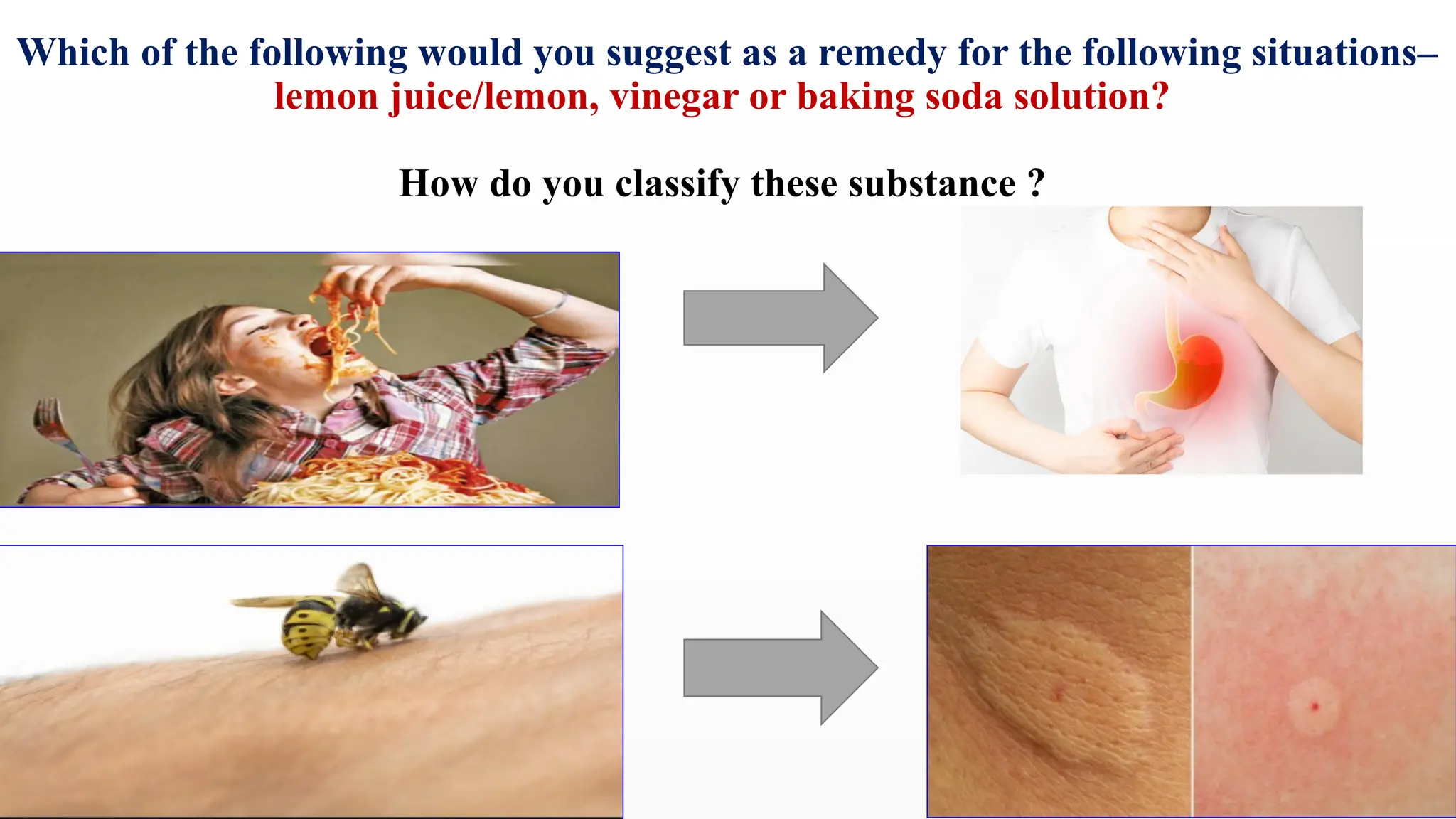
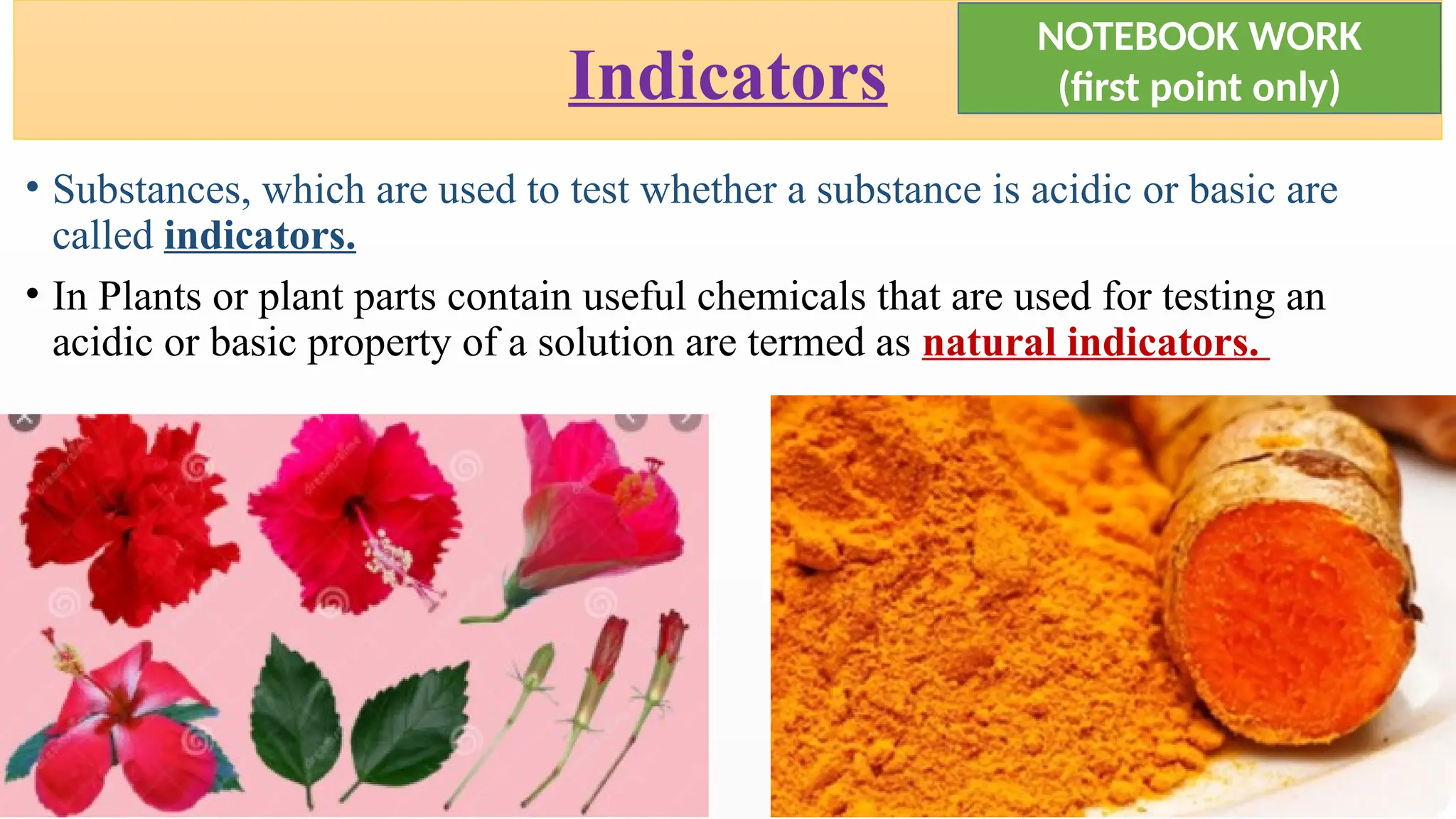
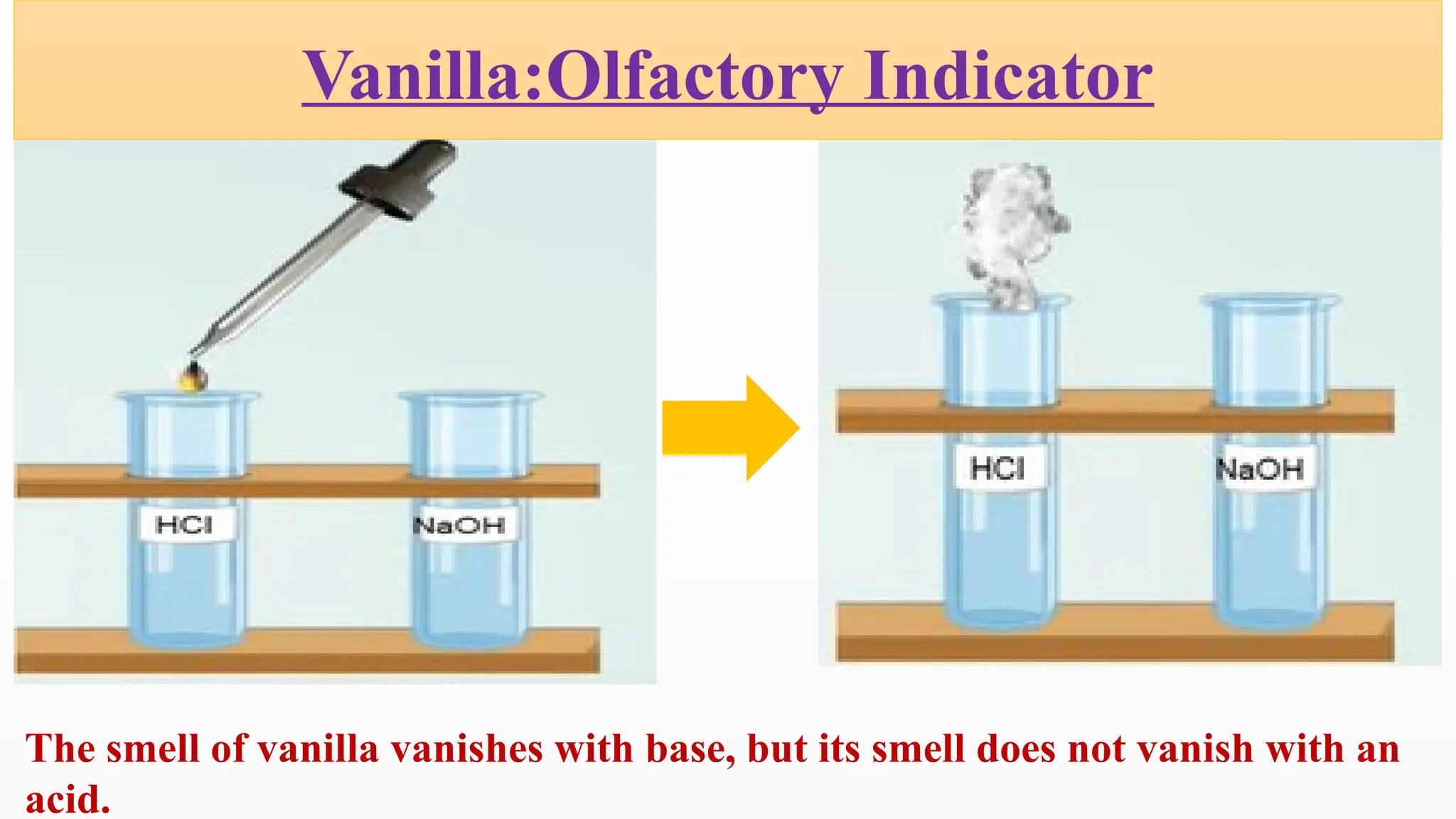
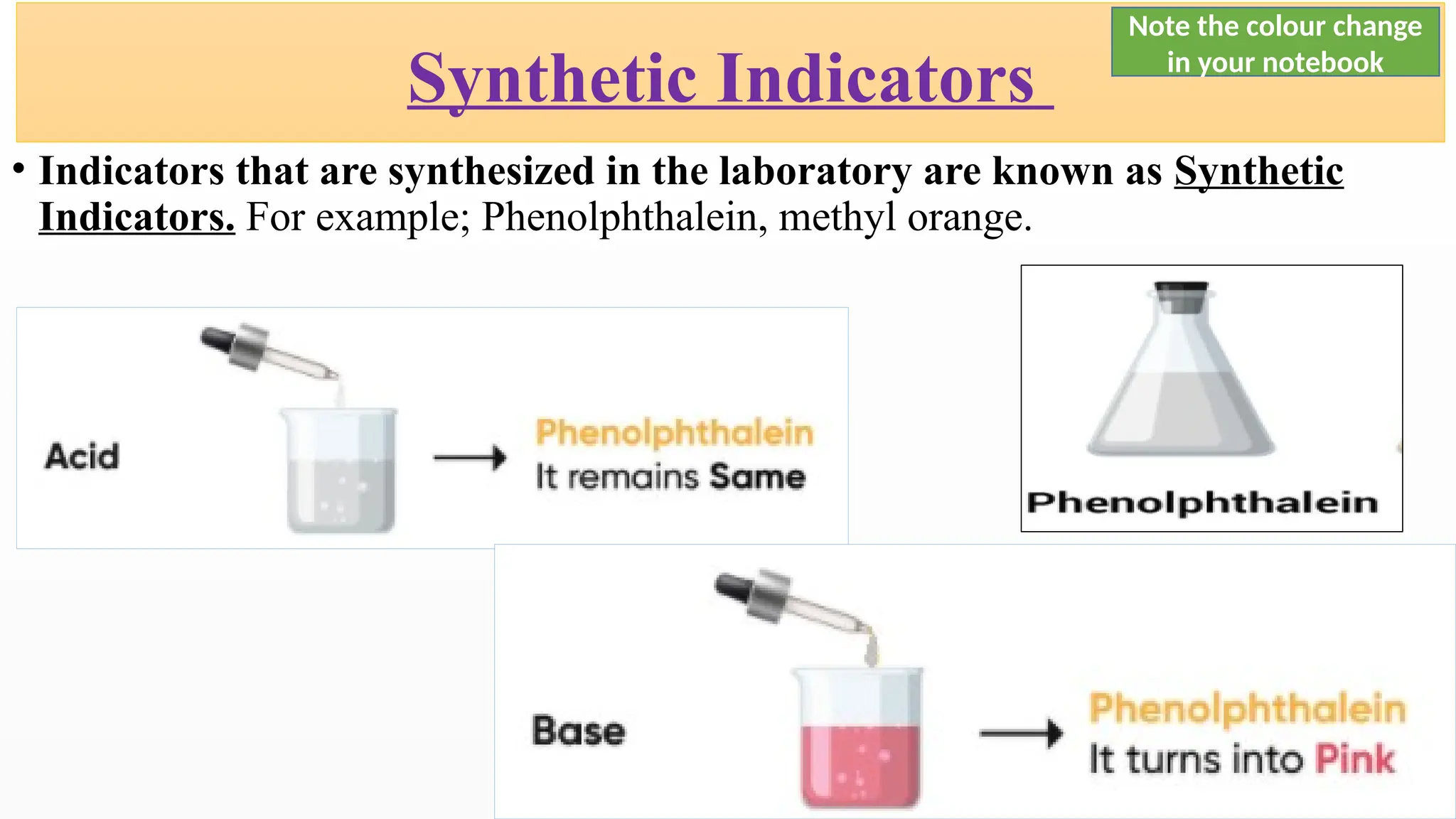
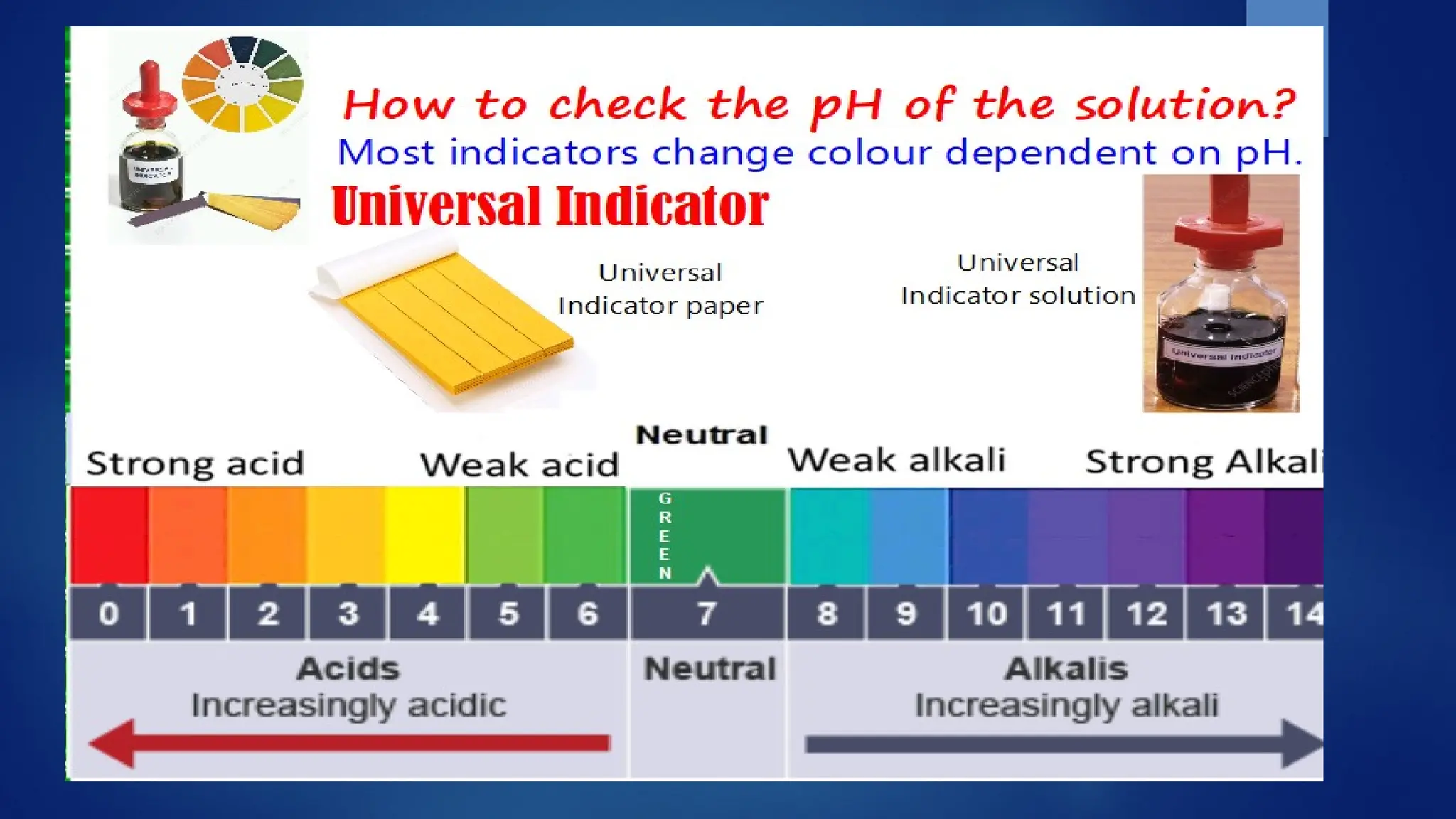
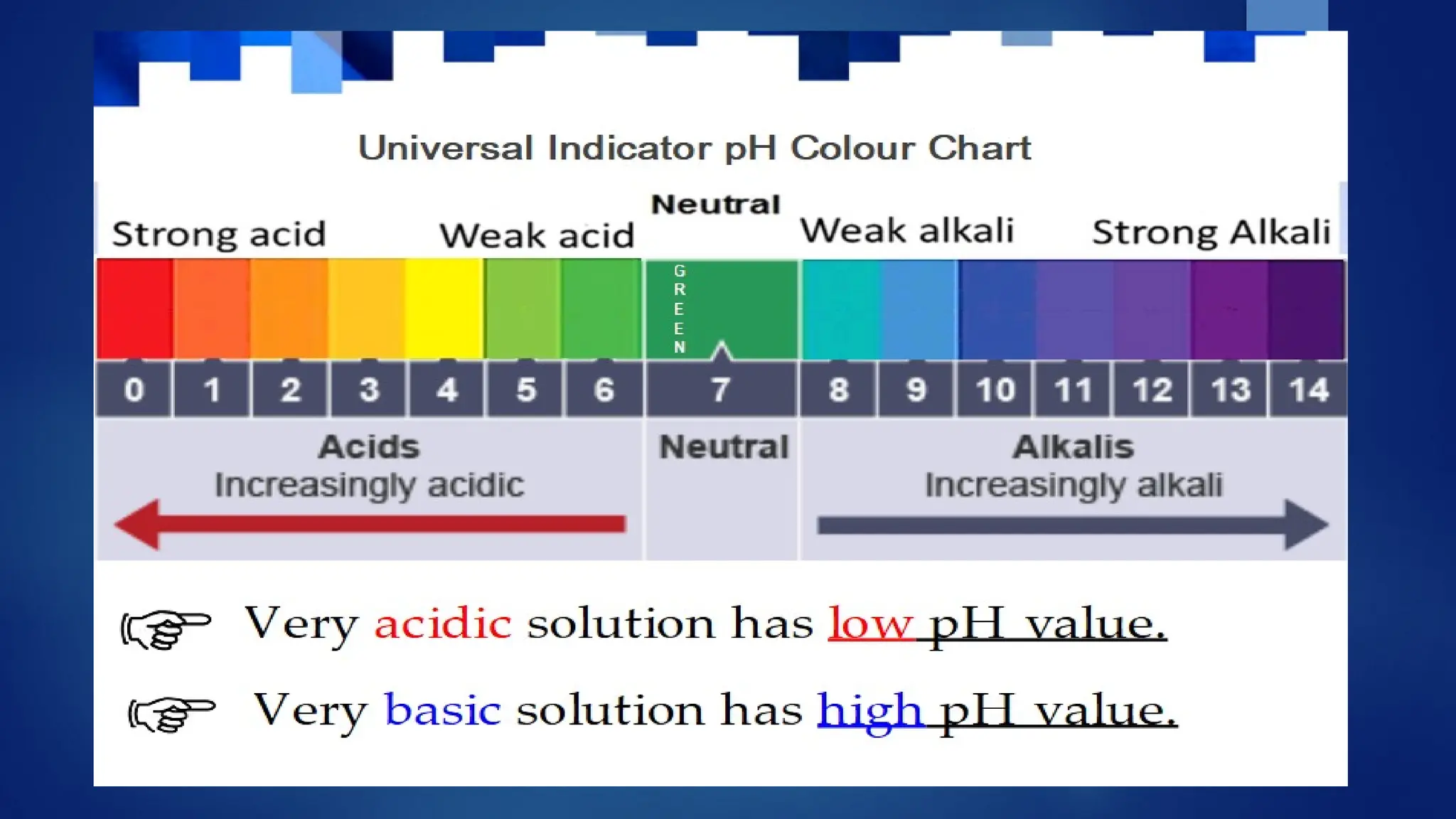
The document covers the classification and properties of acids, bases, and indicators, emphasizing their everyday applications and neutralization reactions. It describes various examples of acids and bases, as well as natural and synthetic indicators used to test pH levels. Learning outcomes include understanding these substances' characteristics and performing relevant tests in practical scenarios.