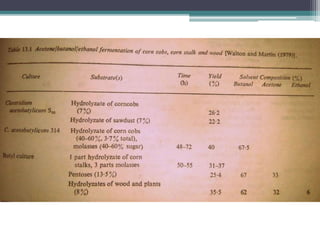
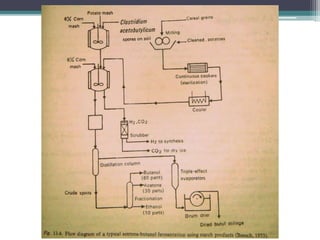
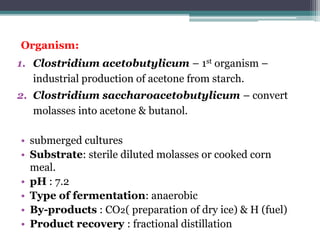
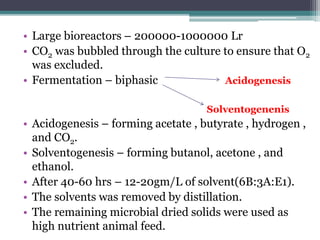
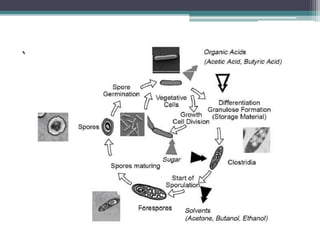
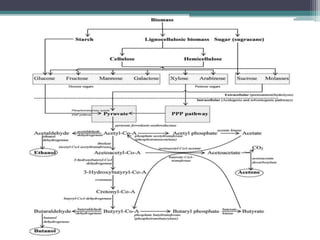
1. Acetone-butanol fermentation is a process that uses Clostridium bacteria to produce acetone and butanol from glucose through anaerobic fermentation.
2. Clostridium acetobutylicum and Clostridium beijerinckii are commonly used species that produce a 3:6:1 ratio of acetone, butanol, and ethanol.
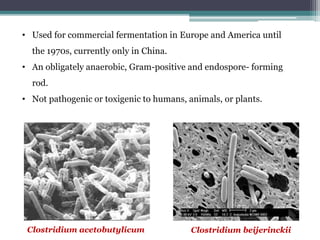
3. Historically this fermentation process was used commercially until the 1970s to produce acetone and butanol, which are used as solvents and in producing synthetic rubbers, lacquers, and explosives.