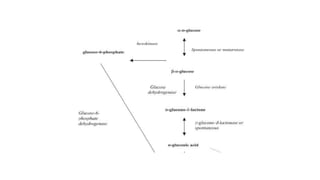
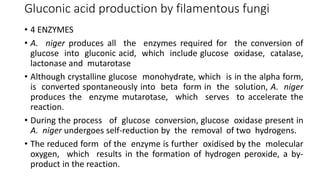
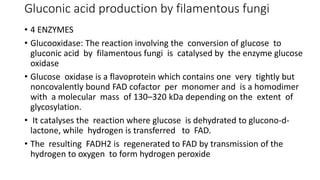
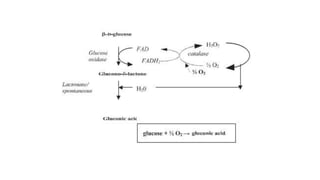
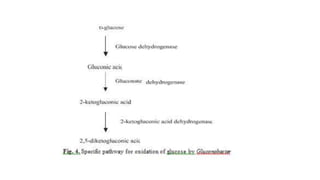
1. Gluconic acid is produced through the oxidation of glucose using enzymes from fungi like Aspergillus niger or bacteria.
2. It has various applications in food, pharmaceuticals, cleaning products due to its properties as a mild acid and metal chelator.
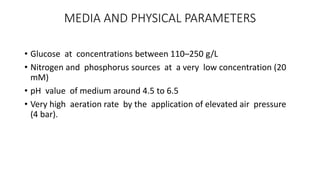
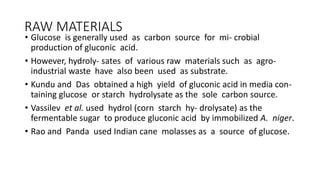
3. Production involves a fermentation process with glucose as the carbon source, followed by recovery of the gluconic acid or calcium gluconate product through crystallization or ion exchange methods.