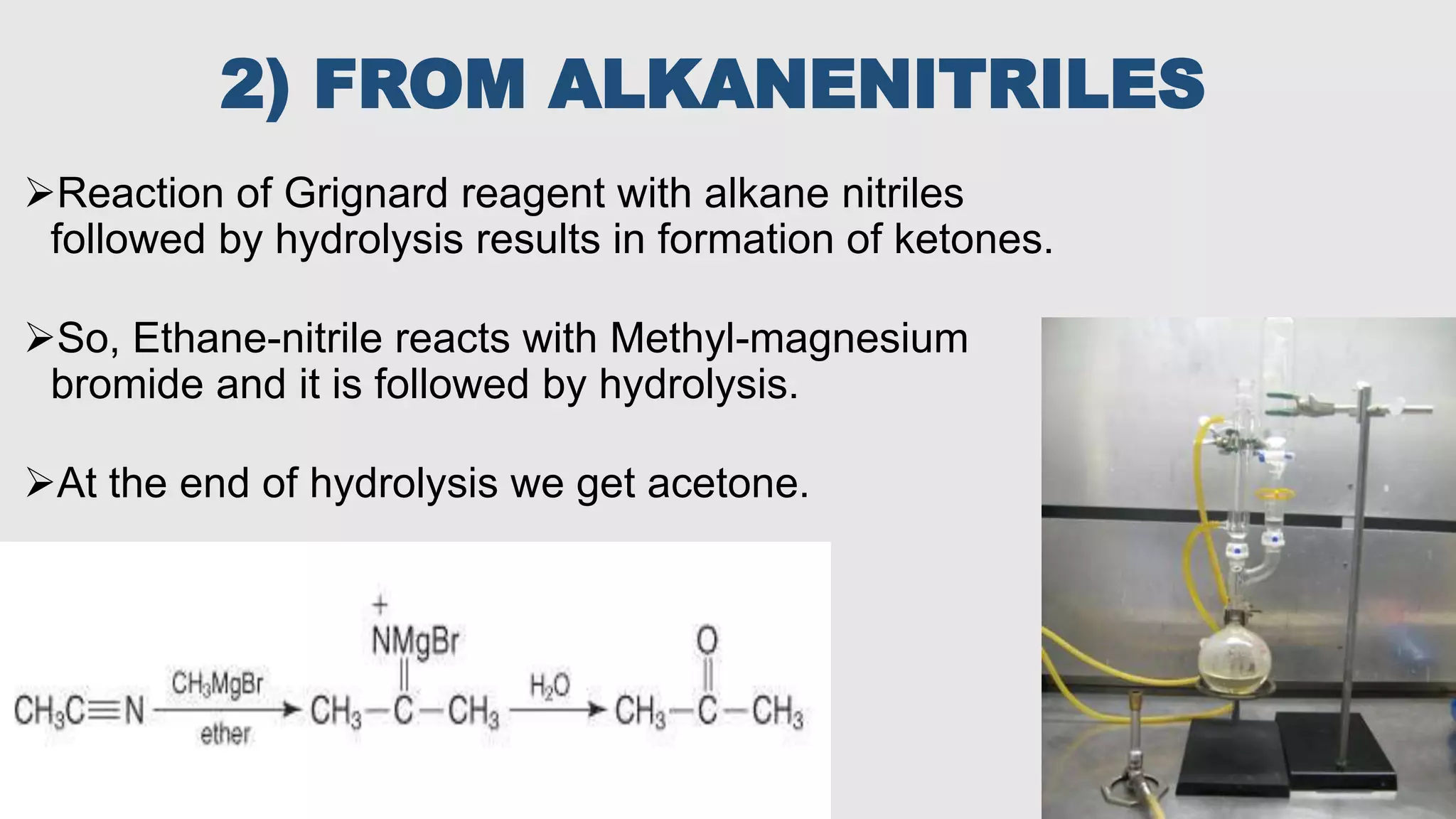
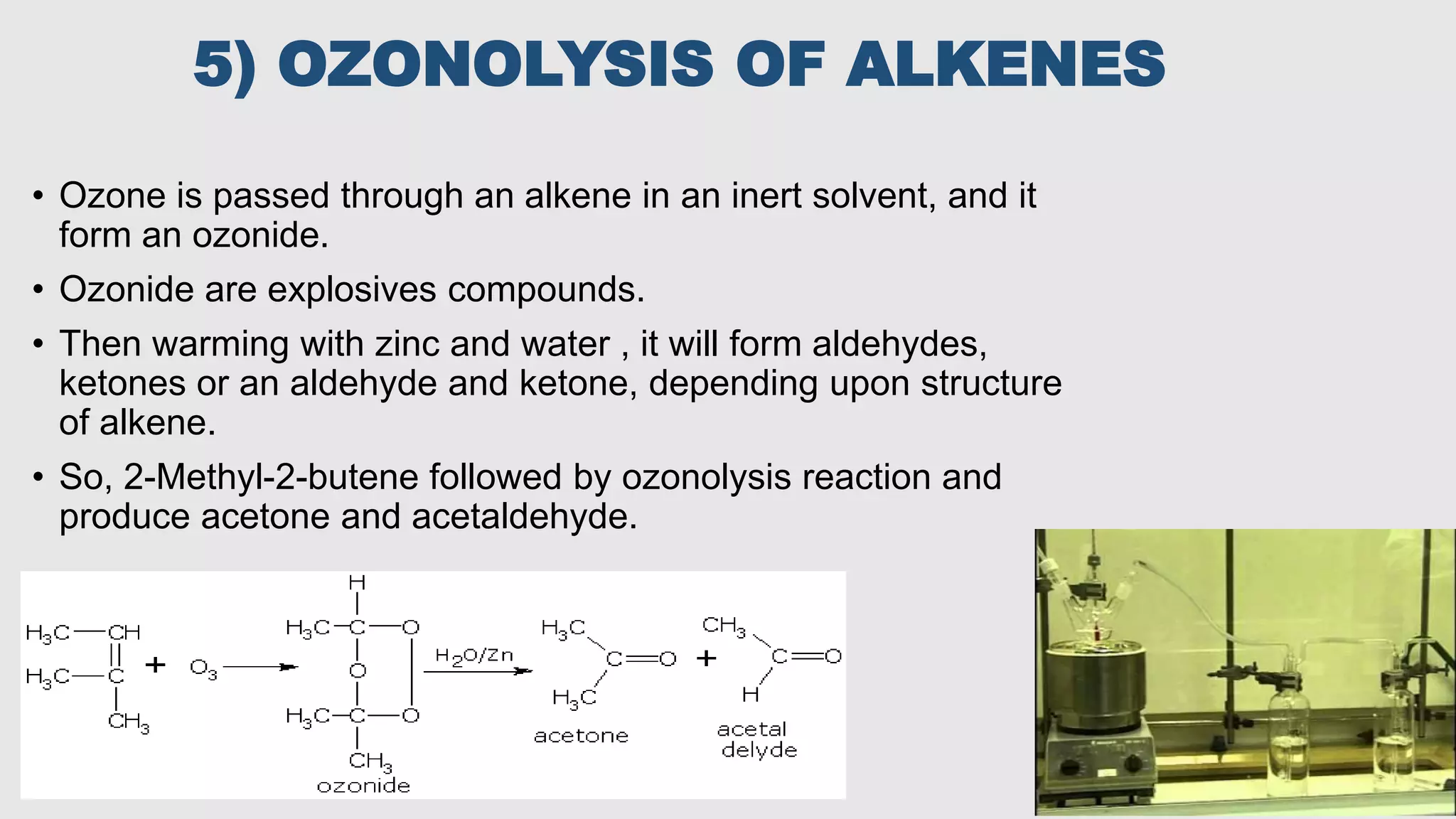
This document discusses various types of chemical solvents including their classification and common uses. It focuses on acetone, describing its structure and various industrial production methods such as the cumene process, fermentation, and oxidation. Acetone is widely used as an industrial and laboratory solvent as well as in products like nail polish remover due to its ability to dissolve many compounds.