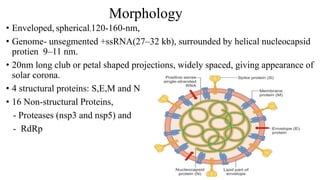
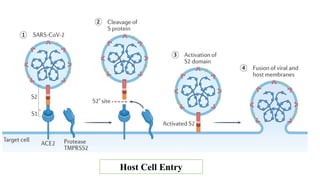
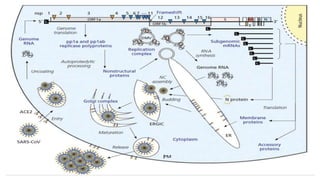
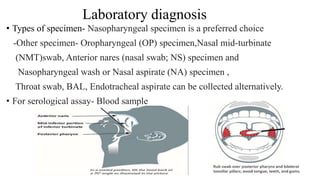
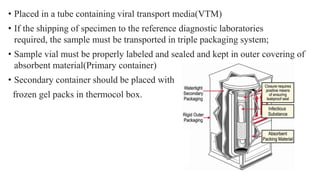
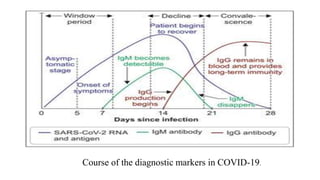
The document outlines the pathogenesis, laboratory diagnosis, management, and prophylaxis of SARS-CoV-2, the virus responsible for COVID-19, first identified in Wuhan, China, in December 2019. It details the virus's morphology, transmission, clinical presentations, diagnostic methods, and management strategies, including treatment and vaccines available in India. The document highlights the epidemiological aspects, including the pandemic's waves and the government's response through lockdowns and health measures.




















![REFERENCES:
• Medical Microbiology – 28th edition, Jawetz, Melnick & Adelberg’s
• Ananthanarayan and Paniker’s Textbook of Microbiology – 8th edition
• Park's textbook of preventive & social medicine, 26TH edition
• Apurba Sanker Sastry, Sandhya Bhat Essentials of Medical Microbiology, 3rd Edition
• Kumar KSR, Mufti SS, Sarathy V, Hazarika D, Naik R. An Update on Advances in COVID-19
Laboratory Diagnosis and Testing Guidelines in India. Front Public Health. 2021 Mar 4;9:568603. doi:
10.3389/fpubh.2021.568603. PMID: 33748054; PMCID: PMC7969786.
• Aleem A, Akbar Samad AB, Vaqar S. Emerging Variants of SARS-CoV-2 and Novel Therapeutics
Against Coronavirus (COVID-19). 2023 May 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls
Publishing; 2023 Jan–. PMID: 34033342.
• Mukim M, Sharma P, Patweker M, Patweker F, Kukkar R, Patel R. Covid-19 Vaccines Available in India.
Comb Chem High Throughput Screen. 2022;25(14):2391-2397. doi:
10.2174/1386207325666220315115953. PMID: 35293291.](https://image.slidesharecdn.com/8-240707071914-3fab2f63/85/8-SARS-COV-2-pptx-LAB-DIAGNOSIS-PROPHYLAXIS-27-320.jpg)
