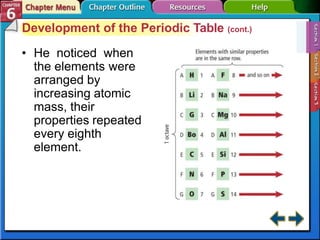
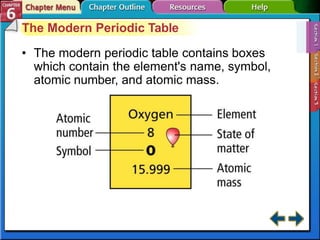
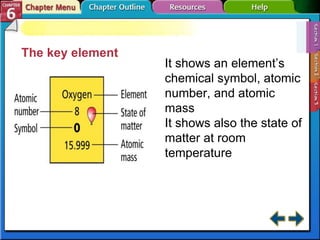
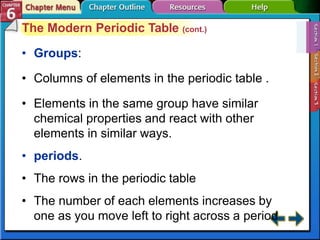
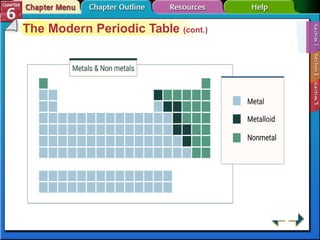
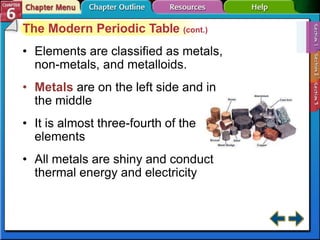
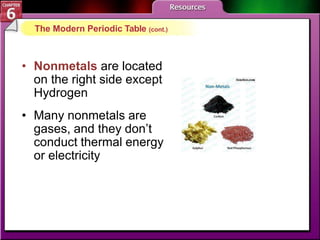
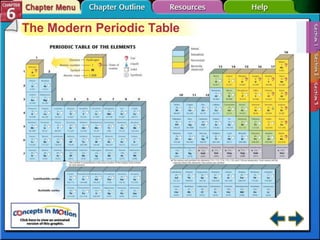
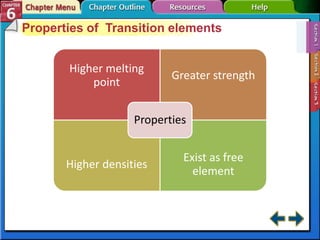
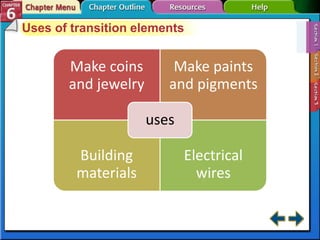
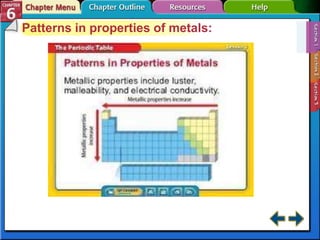
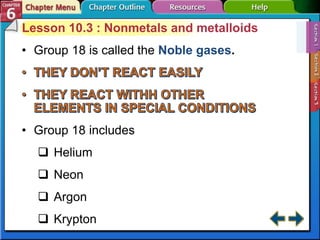
The document traces the development of the periodic table from early lists of elements compiled by scientists like Lavoisier to Mendeleev's groundbreaking periodic table that included predictive properties. It organized elements by atomic mass and left gaps for undiscovered elements, correctly predicting properties of three. Moseley later reorganized the table by atomic number, establishing the modern periodic table's clear periodic trends when arranged by this property. The document also outlines key properties of metals, nonmetals, and metalloids and how they are grouped on the periodic table.