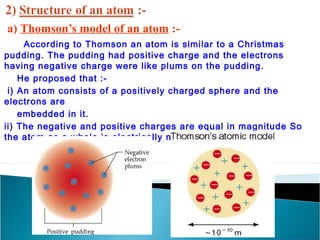
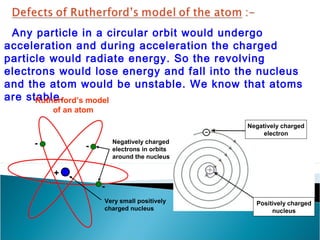
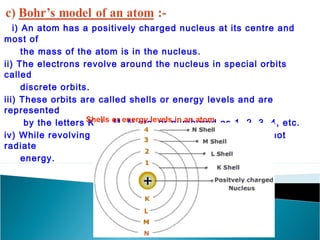
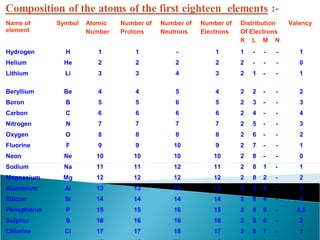
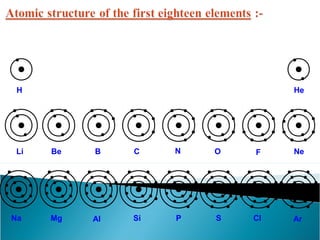
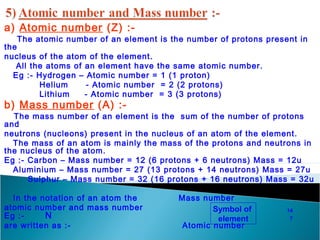
- Atoms contain positively charged protons, uncharged neutrons, and negatively charged electrons. Electrons orbit the small, dense nucleus that contains protons and neutrons.
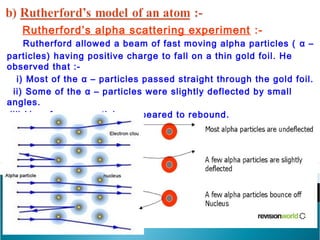
- Rutherford's gold foil experiment showed that the atom consists mostly of empty space, with a small, dense nucleus at the center containing most of the atom's mass.
- Electrons orbit the nucleus in distinct energy levels or shells, allowing atoms to be stable without electrons losing energy through radiation.