To determine the subatomic particles of an atom, one must:
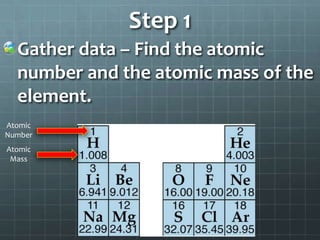
1. Gather the atomic number and mass, with the number representing protons and electrons and mass representing total protons and neutrons.
2. Identify protons as equal to the atomic number.
3. Identify electrons as equal to protons to maintain neutral charge.
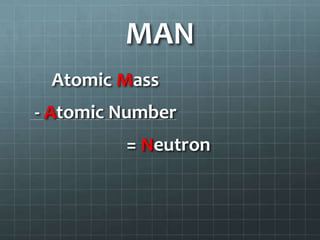
4. Calculate neutrons by subtracting the atomic number from the rounded atomic mass.