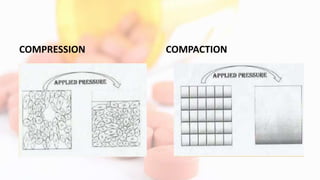
1. Tablet compression involves several mechanisms including particle rearrangement, deformation through elastic or plastic flow, fragmentation, and bonding through mechanical interlocking or intermolecular forces under pressure.
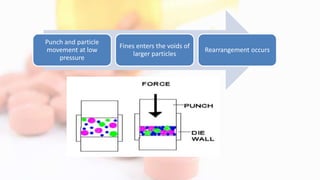
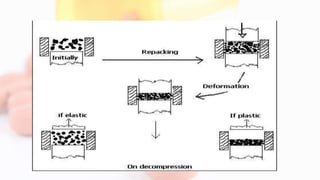
2. As pressure increases, particles first rearrange then deform through elastic and plastic mechanisms, which can lead to fragmentation and new surfaces for bonding.
3. Further increases in pressure cause consolidation through plastic deformation and bonding until ejection, where problems like capping, lamination, or sticking can occur if tablets do not release from the punch properly.