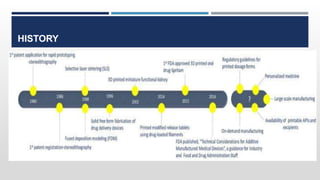
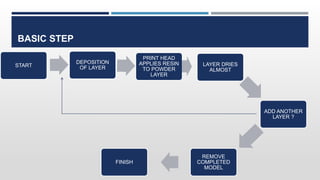
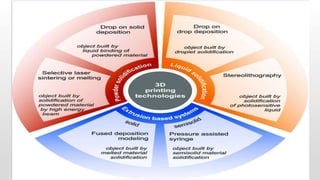
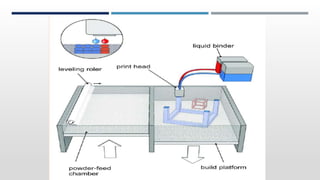
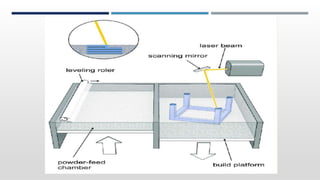
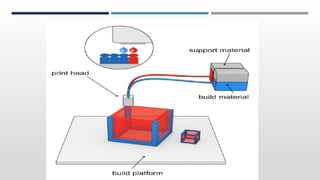
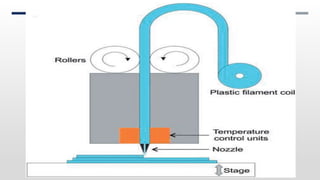
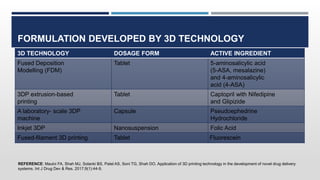
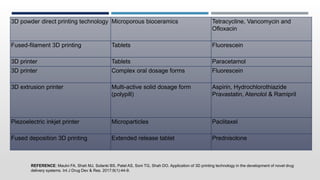
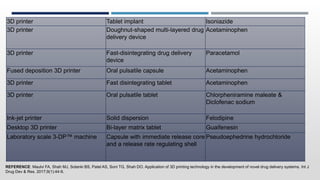
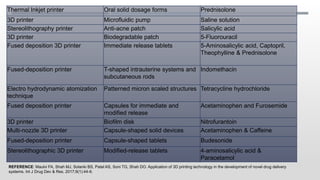
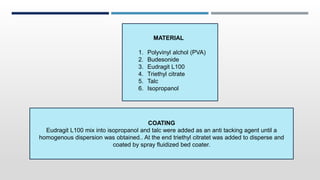
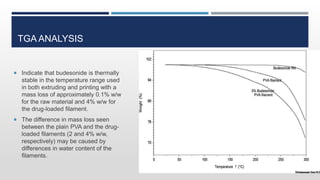
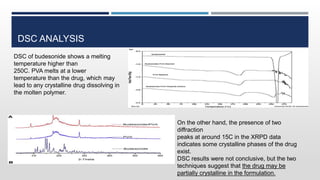
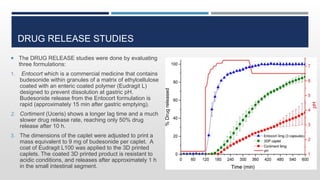
The document discusses 3D printing technology, detailing its definition, history, methods, products, and advantages, particularly in the pharmaceutical sector. Key techniques include powder solidification, liquid solidification, and extrusion-based methods, each with unique processes and applications. The advantages of 3D printing in pharmaceuticals include personalized medication, reduced production costs, and improved efficacy and safety of drug delivery systems.