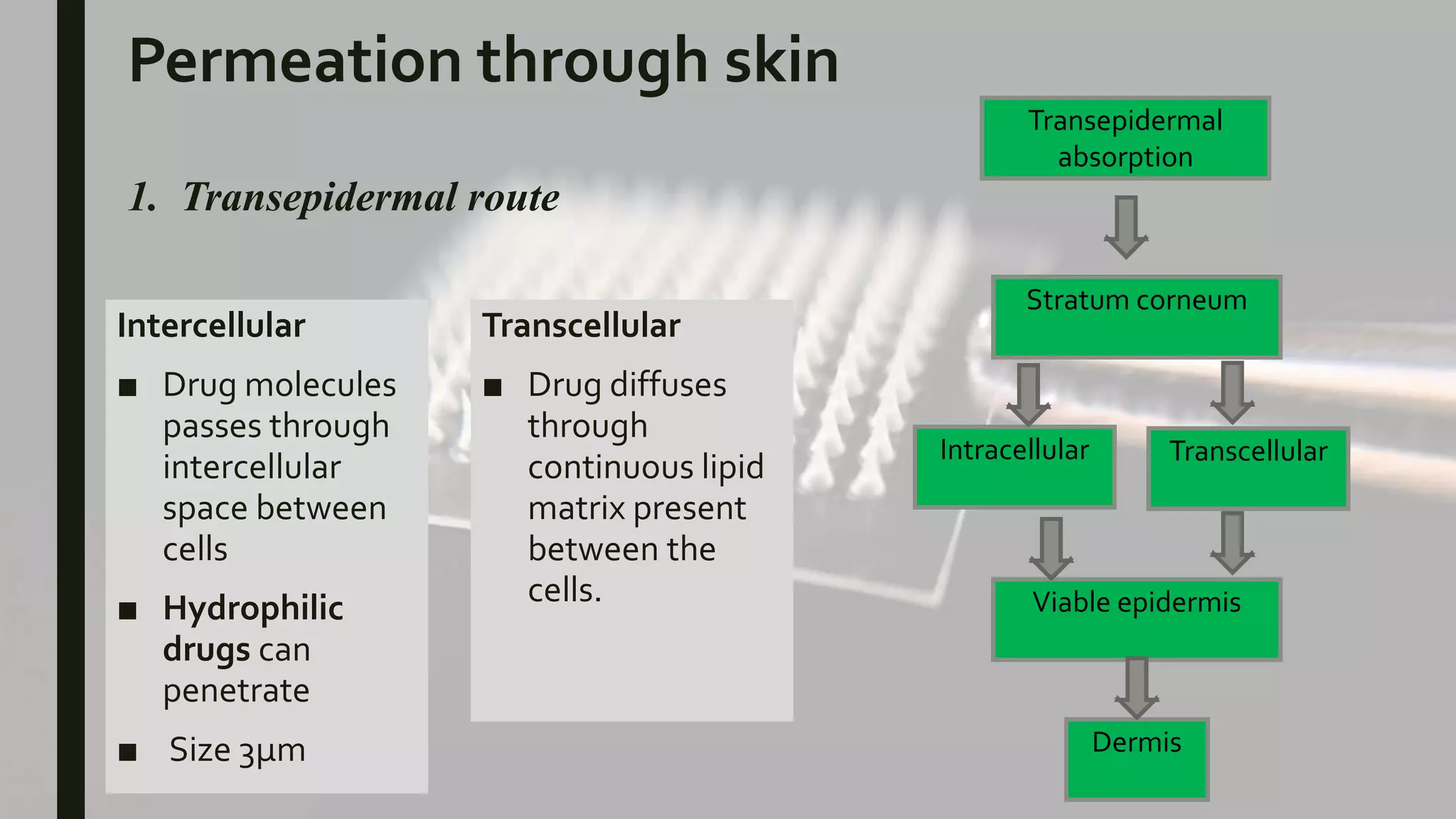
The document discusses transdermal drug delivery systems (TDDS). It provides an overview of TDDS, including their advantages and limitations. It describes the structure of the skin and factors that influence drug permeation. The basic components of TDDS are described, including polymer matrices, drugs, permeation enhancers, pressure-sensitive adhesives, backing layers and release liners. Different types of TDDS patches are outlined, and evaluation methods are summarized, including physicochemical testing and in vitro drug release studies. Examples of marketed TDDS are provided.