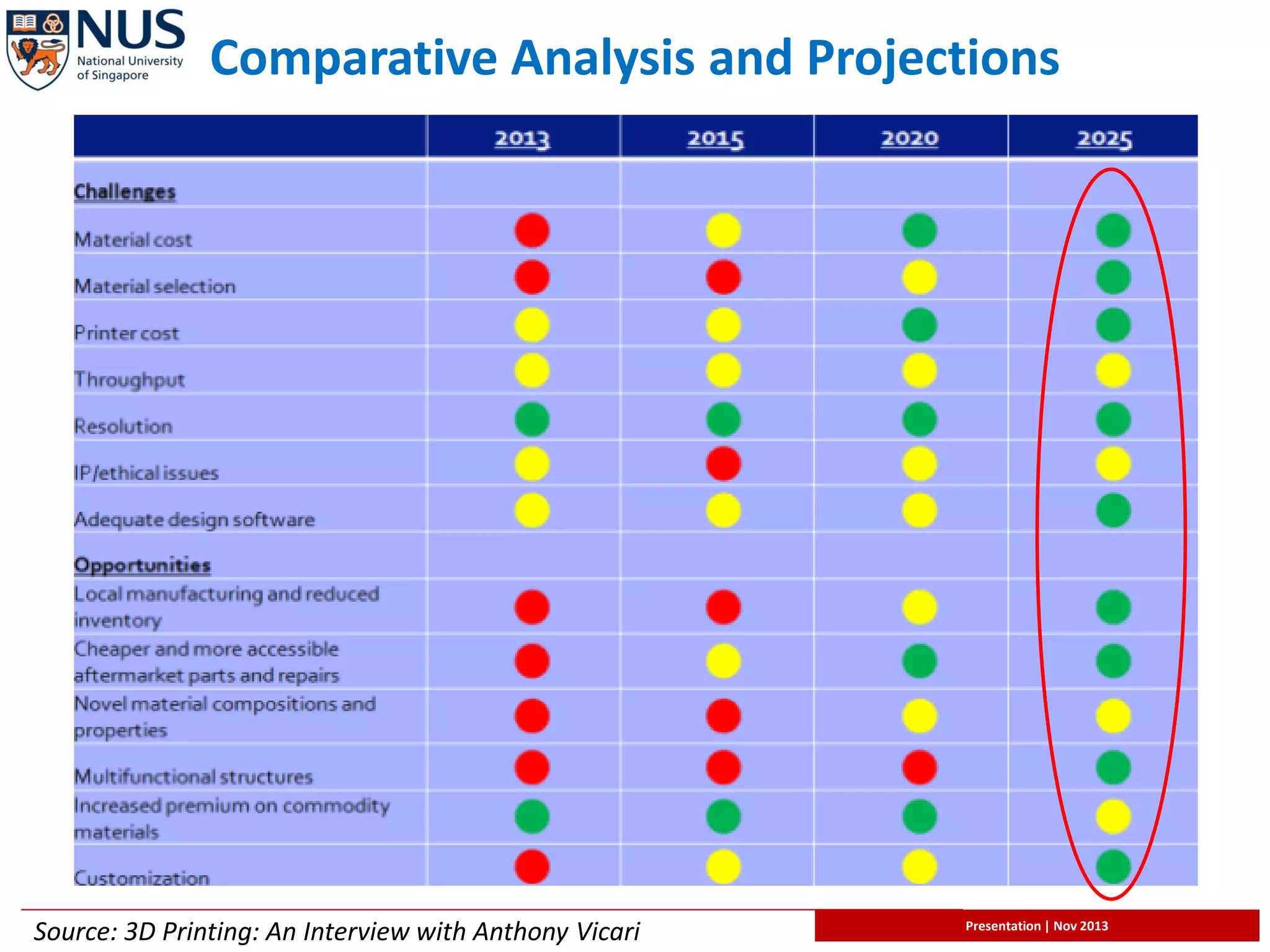
The document provides an overview of 3D printing technology, with a focus on its biological applications, particularly bioprinting for organ creation. It discusses the evolution, performance metrics, and potential future of 3D printing, including its market impact and entrepreneurial opportunities in various industries. Key challenges and advancements in bioprinting, including cost analysis and the necessity for improved technology, are also highlighted.