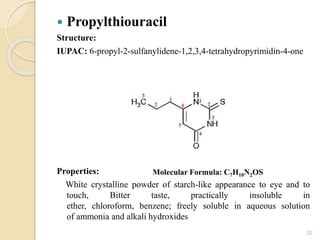
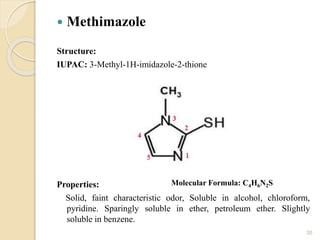
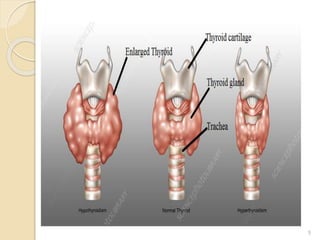
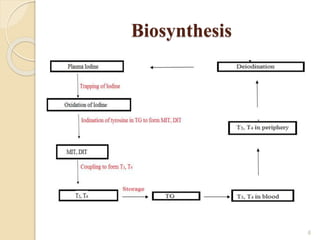
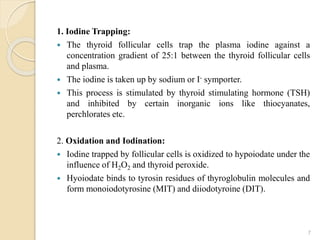
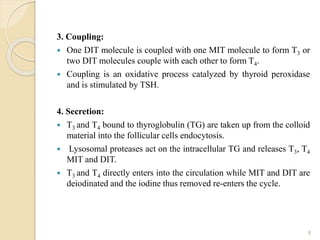
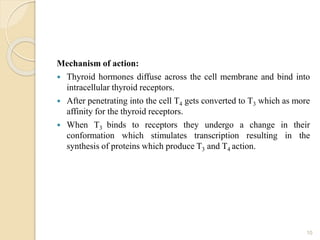
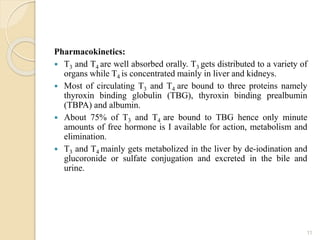
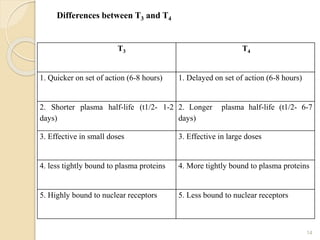
This document provides an overview of thyroid and antithyroid drugs, detailing their biosynthesis, mechanisms of action, pharmacokinetics, therapeutic uses, and adverse reactions. It highlights the functionality of thyroid hormones, explains the conditions of hypothyroidism and hyperthyroidism, and lists various treatments including thioamides and iodides. The document also compares key characteristics of T3 and T4 hormones and emphasizes the relevance of monitoring adverse effects during treatment.









![ L-Thyroxine
Structure:
IUPAC: 2-Amino-3-[4-(4-hydroxy-3,5-diiodophenoxy)-3,5-
diiodophenyl]propanoic acid
Properties:
Solid Crystals, Slightly soluble in water, Insoluble in ethanol, benzene
20
Molecular FormulaC15H11I4](https://image.slidesharecdn.com/4thunit-thyroidandantithyroiddrugs-201105084747/85/4th-unit-thyroid-and-antithyroid-drugs-20-320.jpg)


![ L-Thyronine
Structure:
IUPAC: 2-amino-3-[4-(4-hydroxyphenoxy)phenyl]propanoic acid
Properties:
Solid Crystals, Slightly soluble in water, Insoluble in ethanol, benzene
23
Molecular Formula: C15H15NO4](https://image.slidesharecdn.com/4thunit-thyroidandantithyroiddrugs-201105084747/85/4th-unit-thyroid-and-antithyroid-drugs-23-320.jpg)