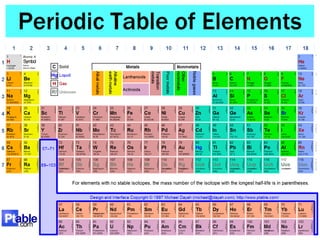
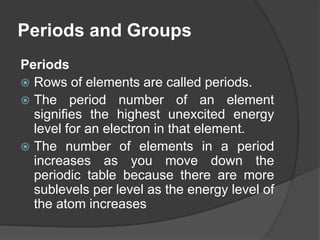
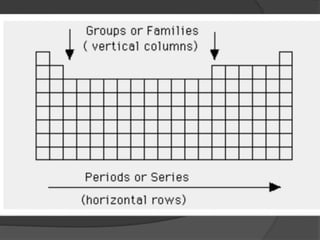
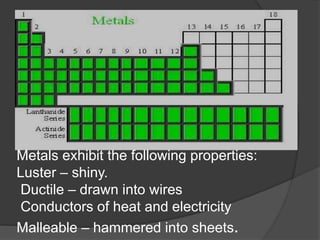
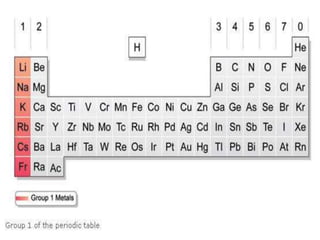
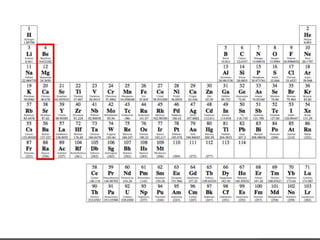
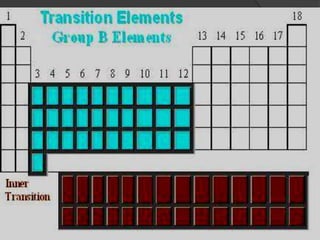
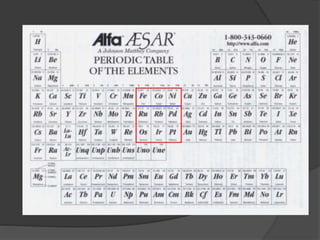
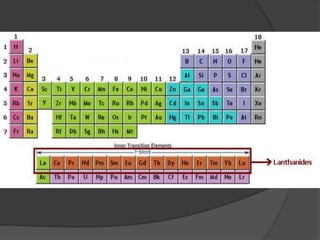
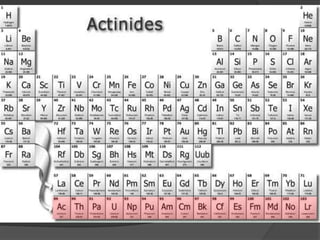
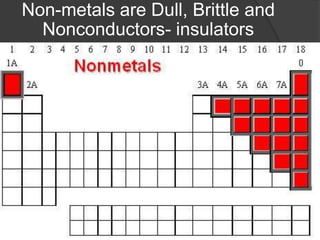
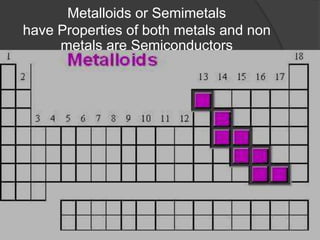
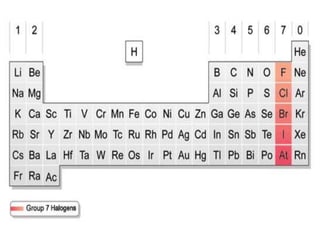
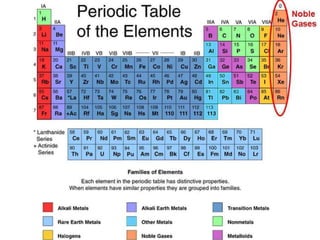
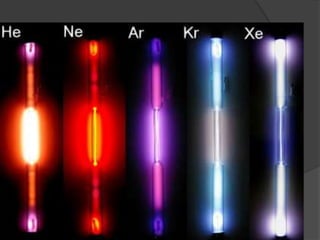
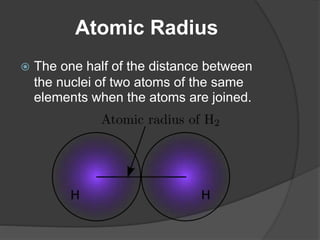
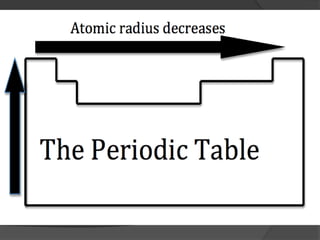
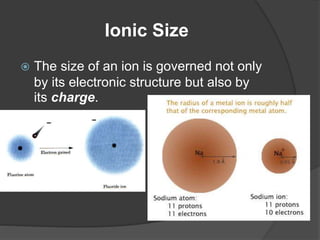
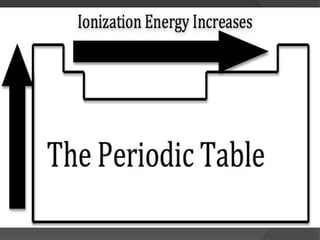
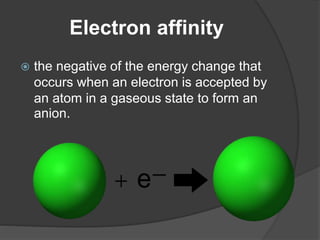
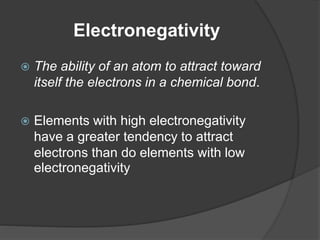
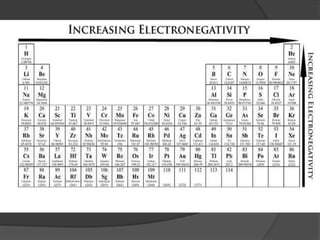
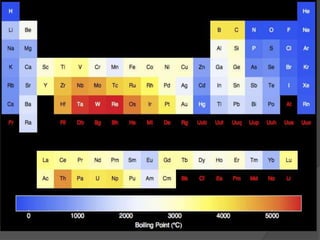
This document discusses the organization and properties of elements in the periodic table. It describes how elements are organized into periods and groups based on their electron configuration. Elements within the same group have similar chemical properties because they have the same number of valence electrons. It also distinguishes between metals, nonmetals, and metalloids, and discusses some representative properties and common elements in various groups of the periodic table.