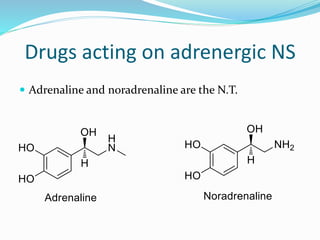
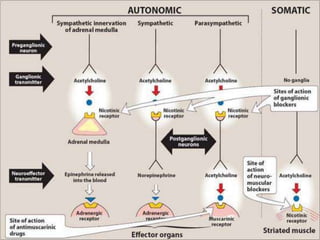
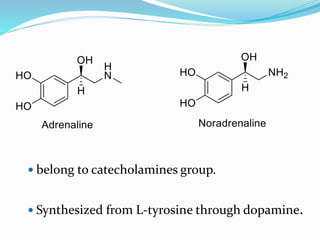
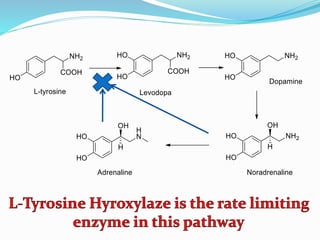
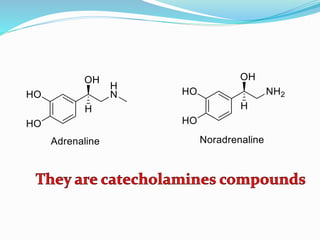
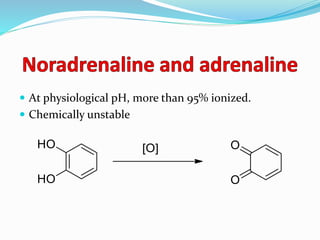
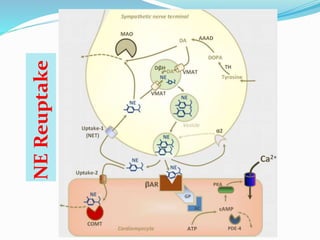
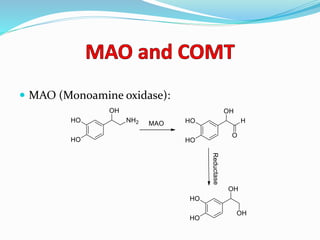
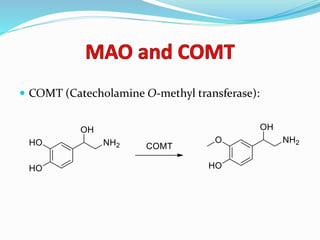
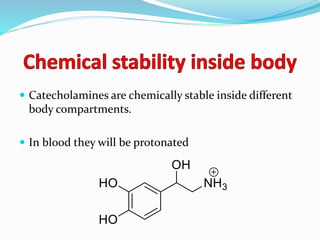
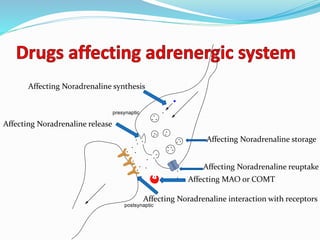
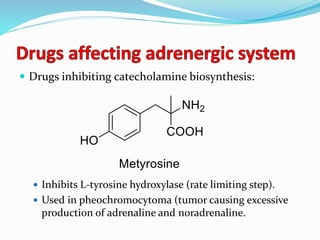
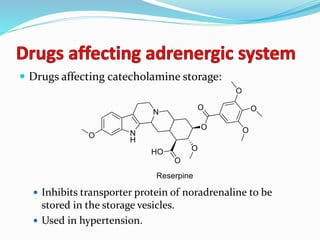
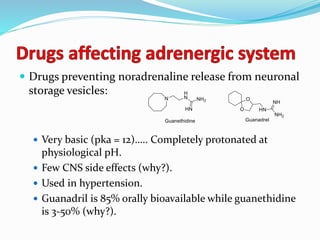
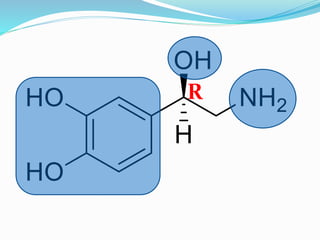
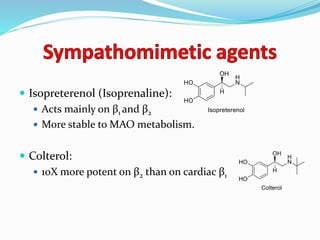
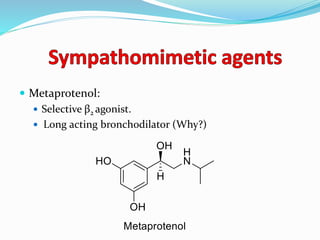
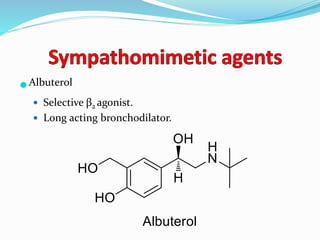
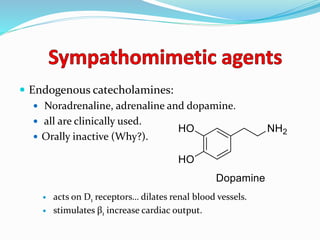
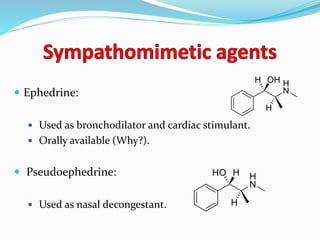
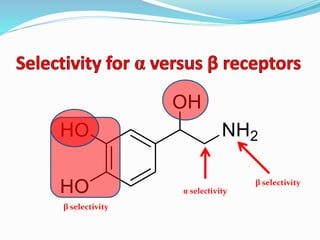
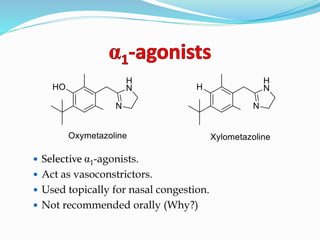
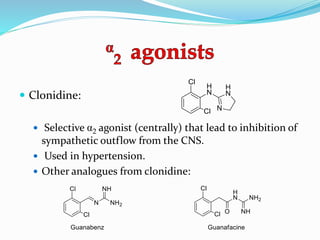
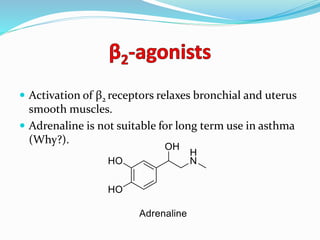
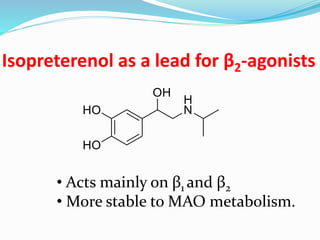
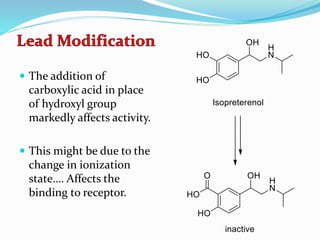
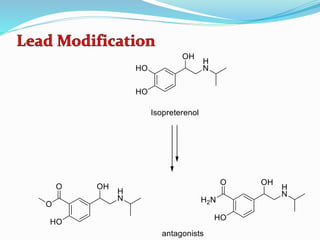
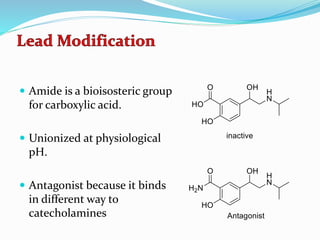
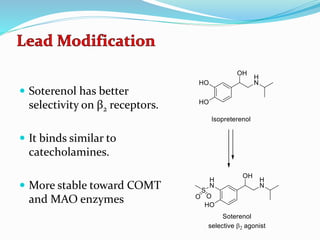
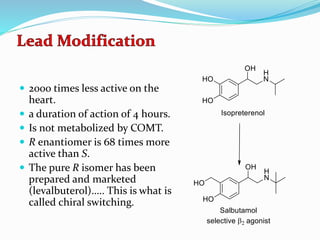
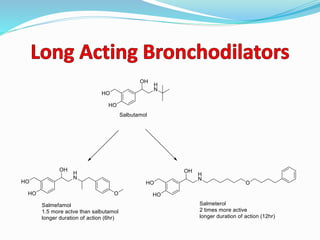
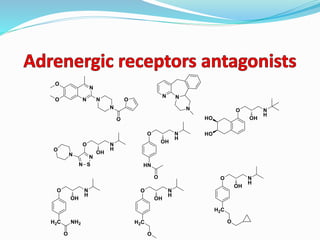
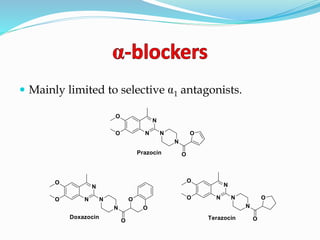
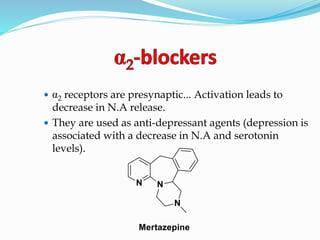
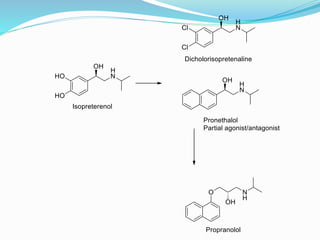
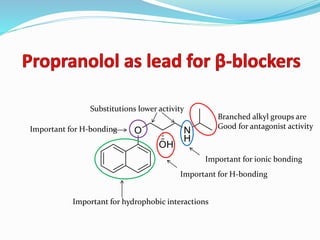
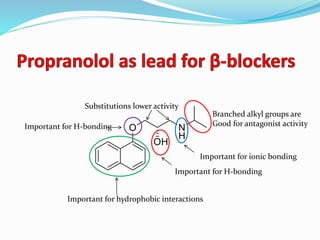
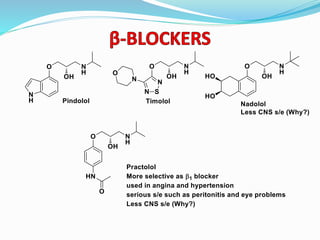
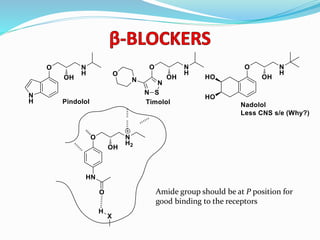
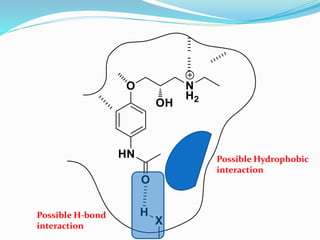
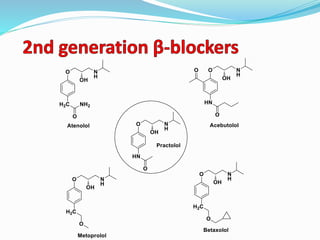
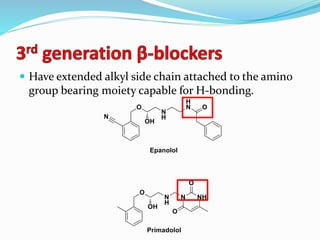
The document discusses the biochemistry and physiology of the sympathetic nervous system, focusing on adrenergic receptors and their agonists and antagonists. It covers the mechanisms of action, structural differences, and clinical applications of various catecholamines and sympathomimetics. Additionally, the document highlights the significance of receptor selectivity in drug design and therapeutic use, particularly in managing diseases like hypertension and asthma.