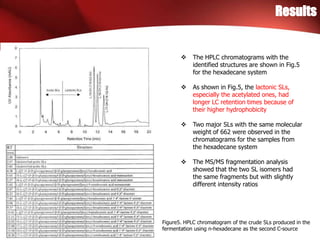
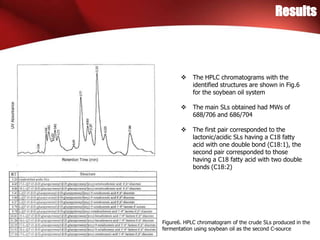
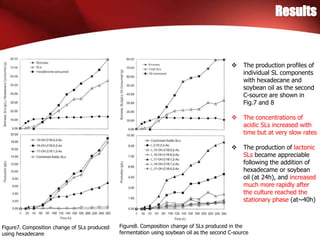
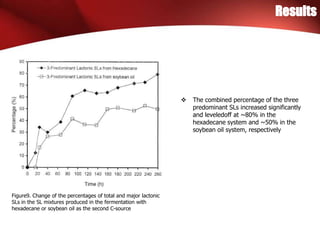
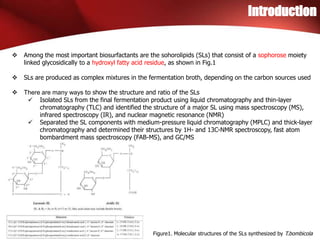
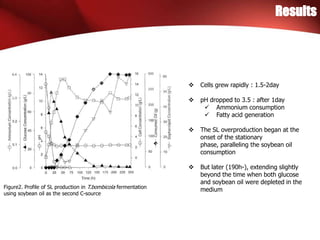
The study investigated the production of sophorolipids (SLs) by Torulopsis bombicola using different lipid precursors such as glucose, hexadecane, and soybean oil. Liquid chromatography-mass spectrometry analysis showed that hexadecane as a precursor produced a cleaner mixture of predominantly diacetylated lactonic SL isomers with palmitate. Soybean oil as a precursor resulted in SLs corresponding to its fatty acid composition. Production of lactonic SLs increased significantly following addition of the lipid precursors and peaked in stationary phase, while acidic SLs increased gradually. Hexadecane provided the highest SL yield and purity compared to soybean oil and glucose precursors.



![Results
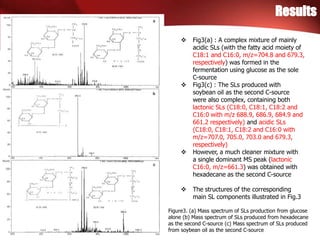
Figure4. MS/MS spectrum from fragmentation of
SLs with m/z=661.3
An example of the MS-MS spectra from
fragmentation is given in Fig4
The molecular ion (M-) had m/z=661.3
m/z=618.5 and 576.8 corresponded to
the ready removal of one and two
acetyl groups, respectively, indication
that the SL was originally diacetylated
A further loss of one glucose from the
de-acetylated structure (m/z=576.8)
led to the fragment ion at m/z=432.7
The loss of both sugars gave the final
fragment ion at
m/z=270.5, corresponding to that from
hydroxyl hexadecanoic acid
The MS-MS spectrum, together with the
structures reported in the
literatures, strongly suggested that the
molecule is the diacetylated lactonic
SL, L-([2’-O-β-D-glucopyranosyl-β-D-
glucopyranosyl] oxy)-hexadecanoic acid
1’-4’’-lactone 6’,6’’-diacetate, as shown
in Fig.3b](https://image.slidesharecdn.com/130629paper-130628235805-phpapp01/85/130629-paper-7-320.jpg)