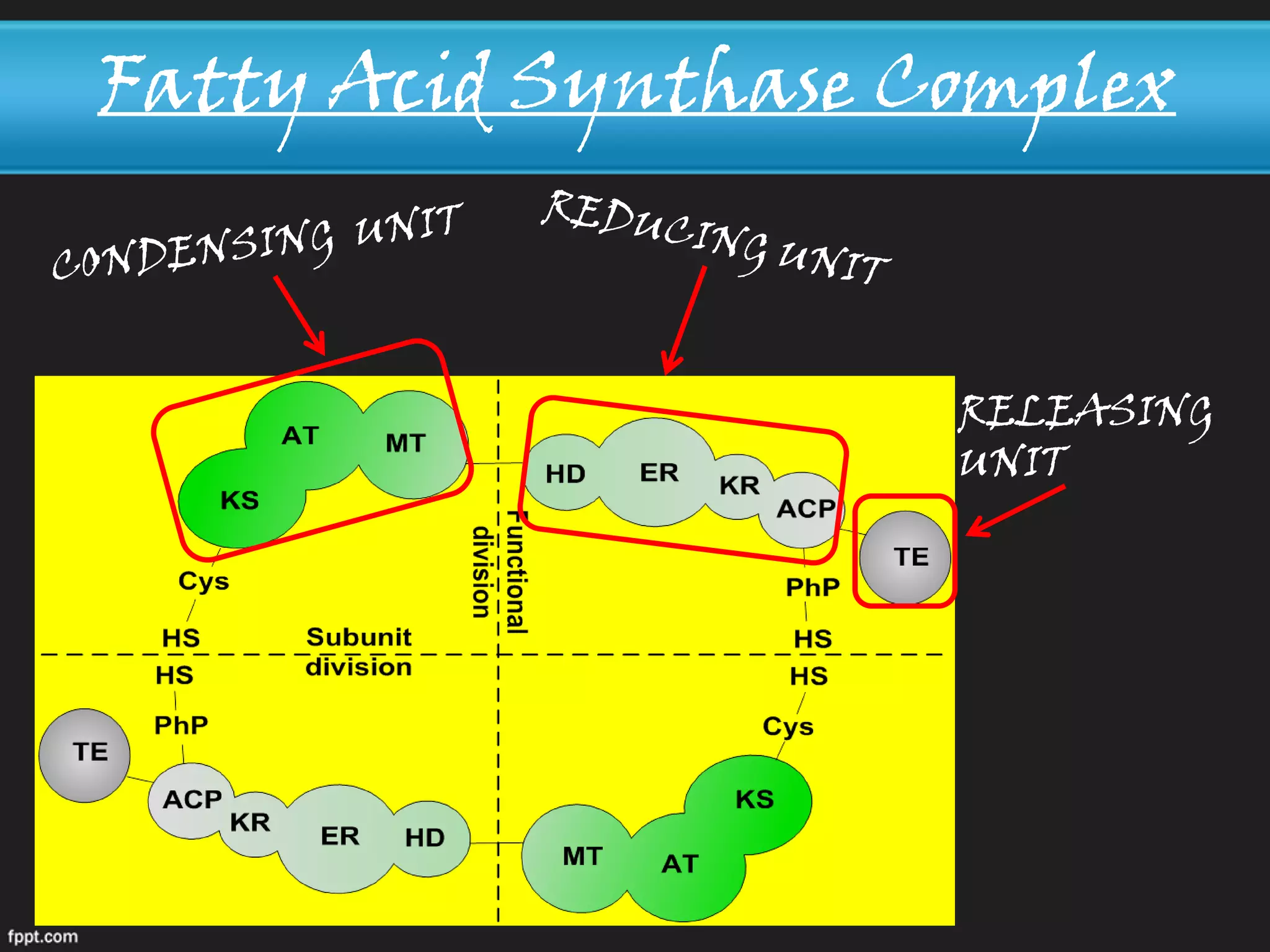
Fatty acid synthesis and degradation occur through different pathways. Synthesis takes place in the cytosol and requires NADPH and acetyl-CoA, adding two carbon units at a time from malonyl-CoA. Degradation occurs in the mitochondria using NADH and FADH2 to split acetyl-CoA units. Fatty acid synthesis is a multi-step process beginning with activation of acetyl-CoA followed by repeated elongation cycles using acetyl-ACP and malonyl-ACP substrates until palmitate is generated, then released by thioesterase.