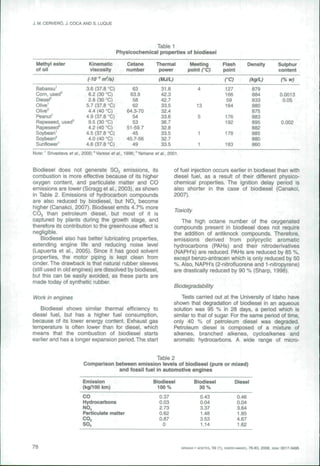
The document discusses the production of biodiesel from vegetable oils through the transesterification of triglycerides, which involves displacing glycerine using low molecular weight alcohols. It reviews both the catalytic and enzymatic pathways for biodiesel synthesis, noting the benefits of using ethanol from renewable sources. Additionally, it compares the properties and emissions of biodiesel to petroleum diesel, highlighting biodiesel's environmental advantages and challenges in production.