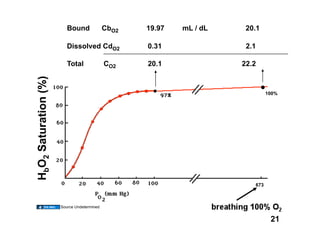
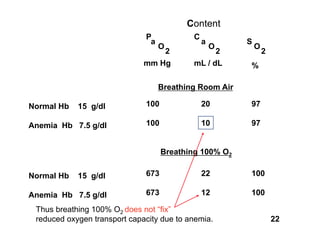
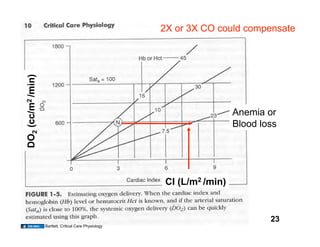
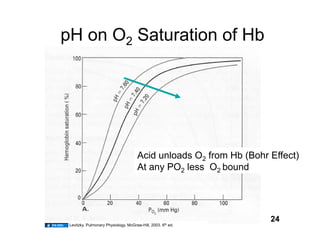
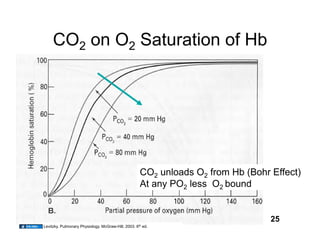
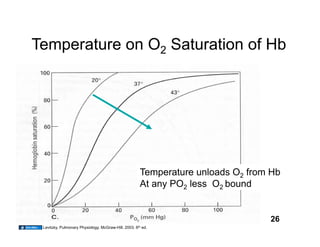
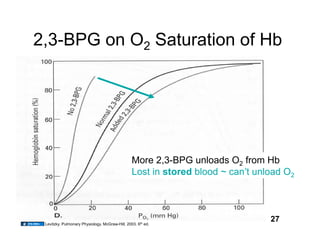
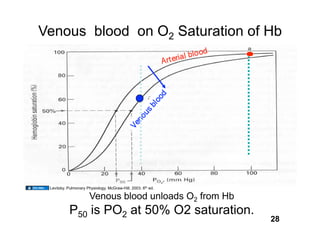
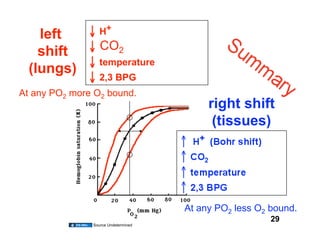
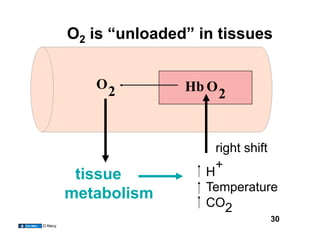
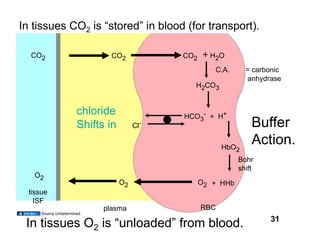
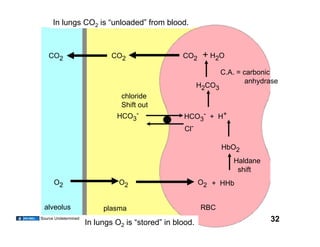
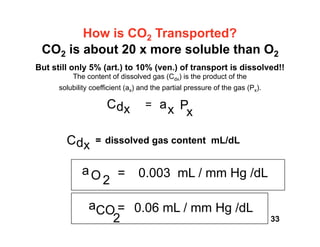
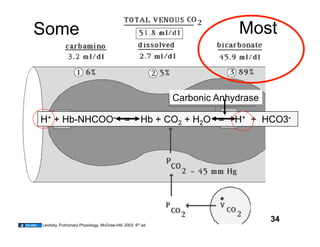
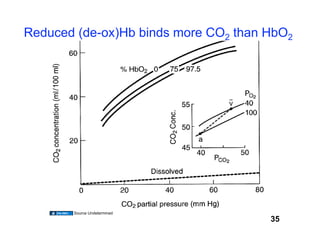
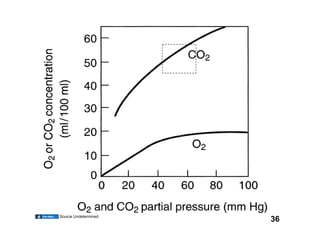
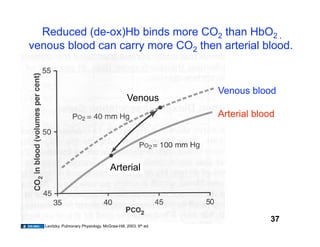
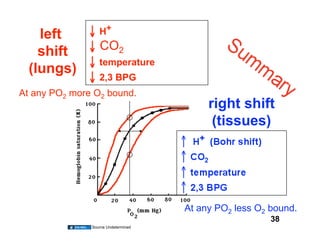
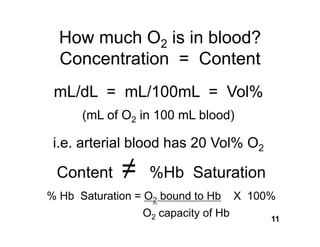
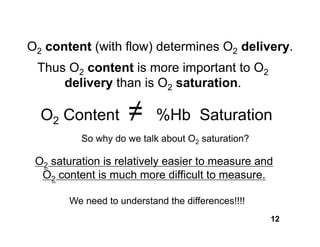
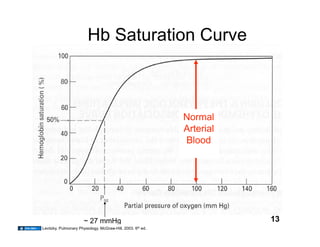
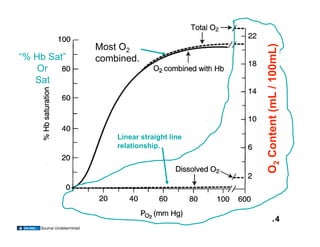
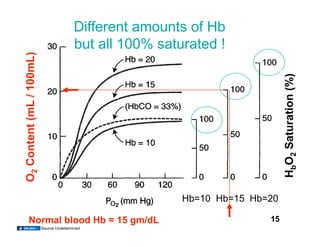
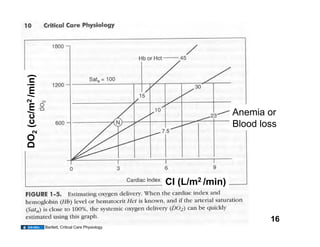
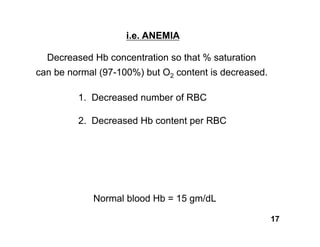
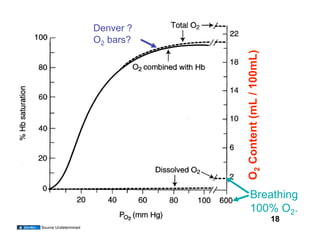
This document discusses the transport of oxygen (O2) and carbon dioxide (CO2) in the blood, including the roles of hemoglobin and carbonic anhydrase, and how various factors affect oxygen delivery. It highlights critical concepts such as the oxygen-hemoglobin saturation curve, the impact of anemia on oxygen content, and the biochemical mechanisms of gas transport. The material is intended for educational purposes and provides guidance on copyright and usage rights.




![Hemoglobin Bound Oxygen Content mL O2 /dL
Cb = S O [ Hb ] Hb s
O2 2
S O = % Hb saturation = O2 bound to Hb X 100%
O2 capacity of Hb
[ Hb ] = hemoglobin content gm/dL blood
Hb s = saturated Hb O2 content mL O2 /gm Hb
Hbs = 1.36 mL O2 /gm Hb = O2 capacity
19](https://image.slidesharecdn.com/111808-ldalecy-o2co2transport-110613152447-phpapp02/85/11-18-08-a-O2-and-CO2-Transport-19-320.jpg)
![Typical Arterial Blood Oxygen Content
P = 100 mm Hg S = 97% [ Hb ] = 15 gm/dL
O O
2 2
Dissolved O
Cd a
O2 = O P
O2
= 0.0031 x 100 = 0.31 mL / dL
2
Bound O
C = S [ Hb ] Hb = 0.97 x 15 x 1.36 = 19.79 mL / dL
b O s
O 2
2
Total Oxygen Content
Cd C
O2 + b = 0.31 + 19.79 = 20.1 mL / dL
O
2
20](https://image.slidesharecdn.com/111808-ldalecy-o2co2transport-110613152447-phpapp02/85/11-18-08-a-O2-and-CO2-Transport-20-320.jpg)