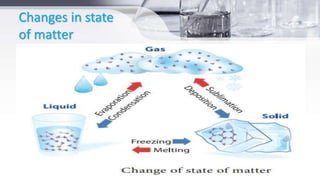
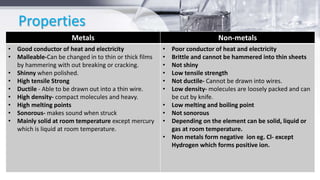
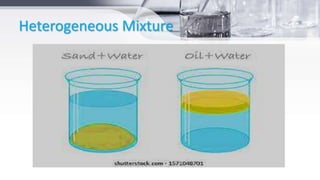
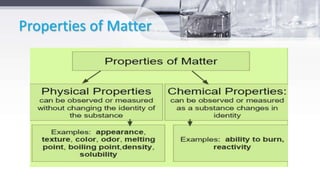
This document provides an introduction to general chemistry concepts. It begins with defining chemistry and discussing its branches, including analytical chemistry, physical chemistry, organic chemistry, and inorganic chemistry. It then discusses the importance of chemistry for nurses, focusing on understanding drug composition and properties, diagnosis, disease mechanisms, and sterilization. The document also defines matter and the three states of matter - solid, liquid, and gas. It provides examples and descriptions of the properties and changes between each state. Finally, it classifies matter as either pure substances like elements and compounds, or impure substances like mixtures, and provides brief definitions of each.