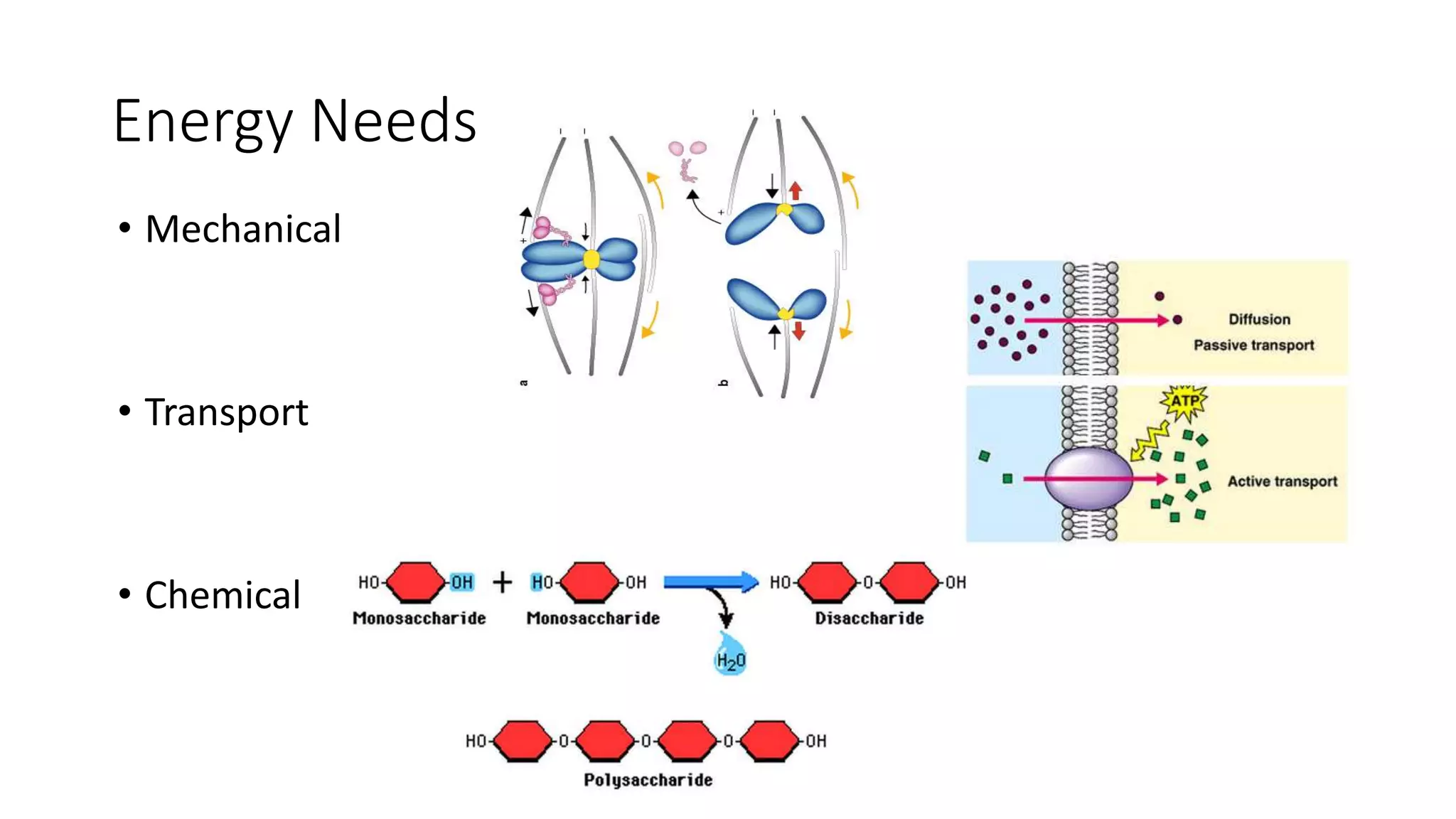
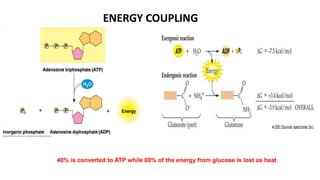
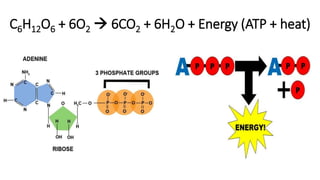
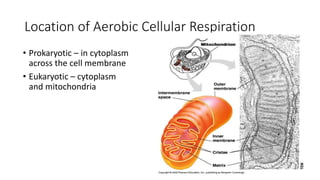
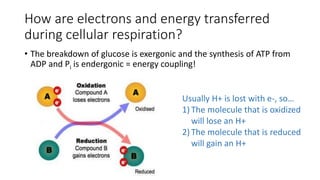
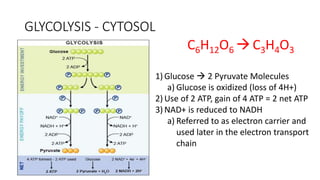
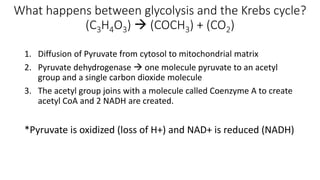
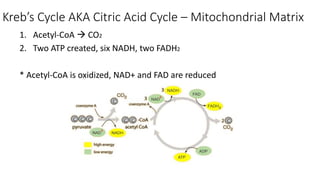
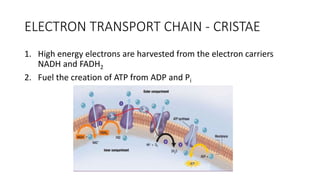
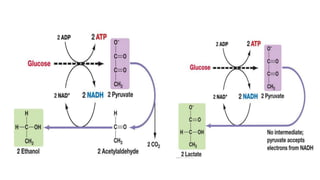
Cellular respiration involves the breakdown of glucose to extract energy in the form of ATP. During aerobic respiration, glucose is broken down through glycolysis, the Krebs cycle, and the electron transport chain. This produces approximately 38 ATP per glucose molecule. However, 60% of the energy from glucose is lost as heat. When oxygen is unavailable, fermentation allows glycolysis to continue and produce some ATP, but much less than during aerobic respiration.