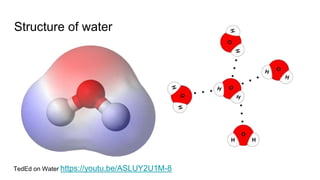
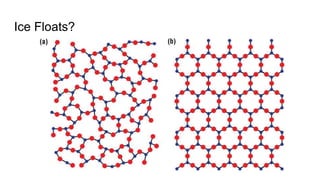
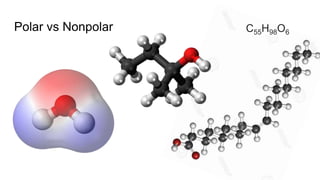
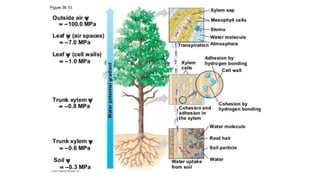
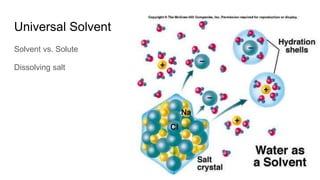
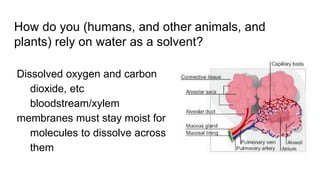
The document discusses the properties and importance of water. It describes water's polarity allows it to have adhesive and cohesive properties which are important for trees. Water is a universal solvent that can dissolve many polar and non-polar substances like sugar, salt, and molecules that are essential for cellular functions and transport in living things. As a solvent in blood and sap, water enables metabolic reactions and movement of dissolved materials throughout organisms.