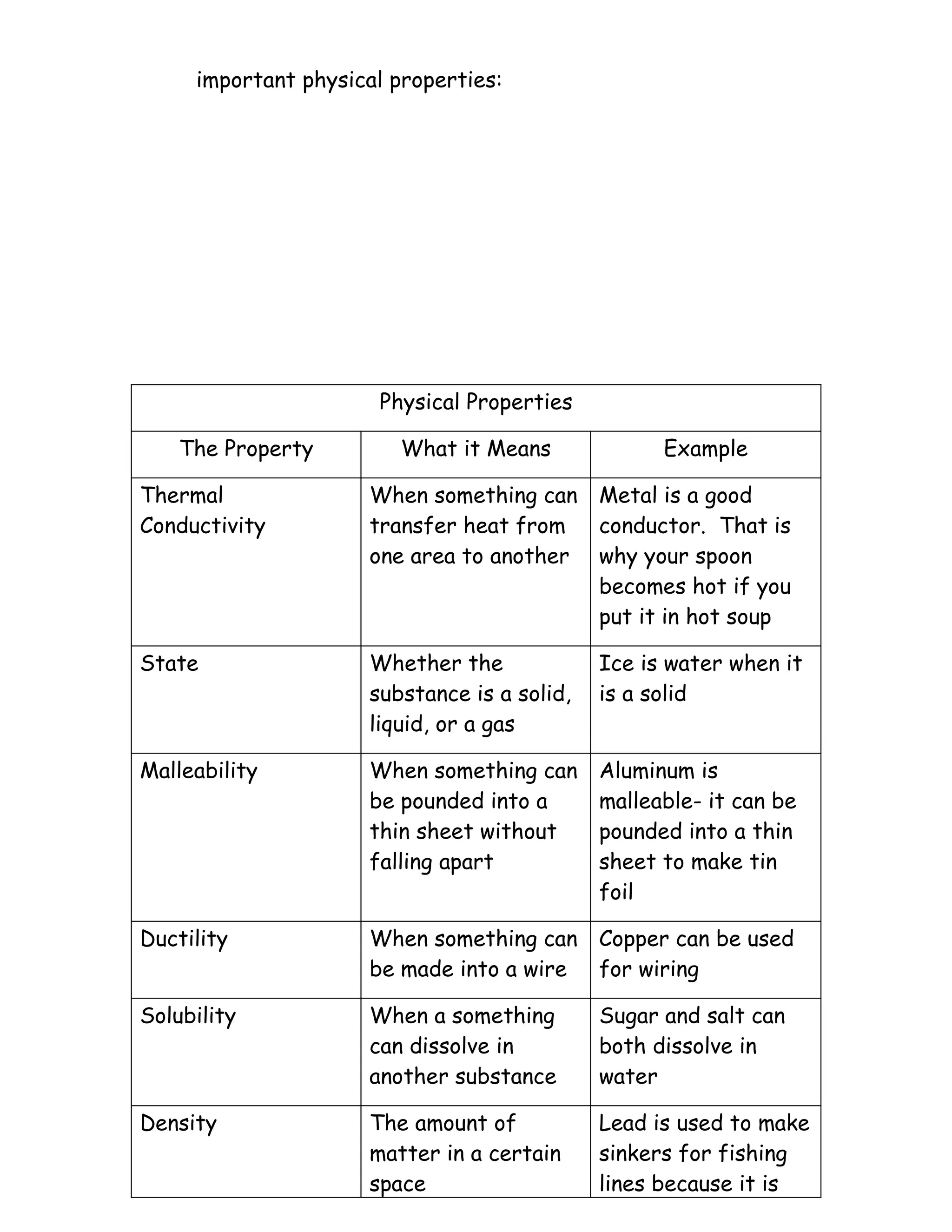
The document discusses the properties of matter. It defines matter as anything that has volume and mass. It describes volume as the amount of space an object takes up and mass as the amount of matter in an object. It distinguishes between mass, which is a measure of the amount of matter, and weight, which is the gravitational force on an object. It then discusses physical properties like state, density, and thermal conductivity, and chemical properties like flammability and reactivity. It contrasts physical changes, which change physical properties but not identity, with chemical changes, which form new substances.