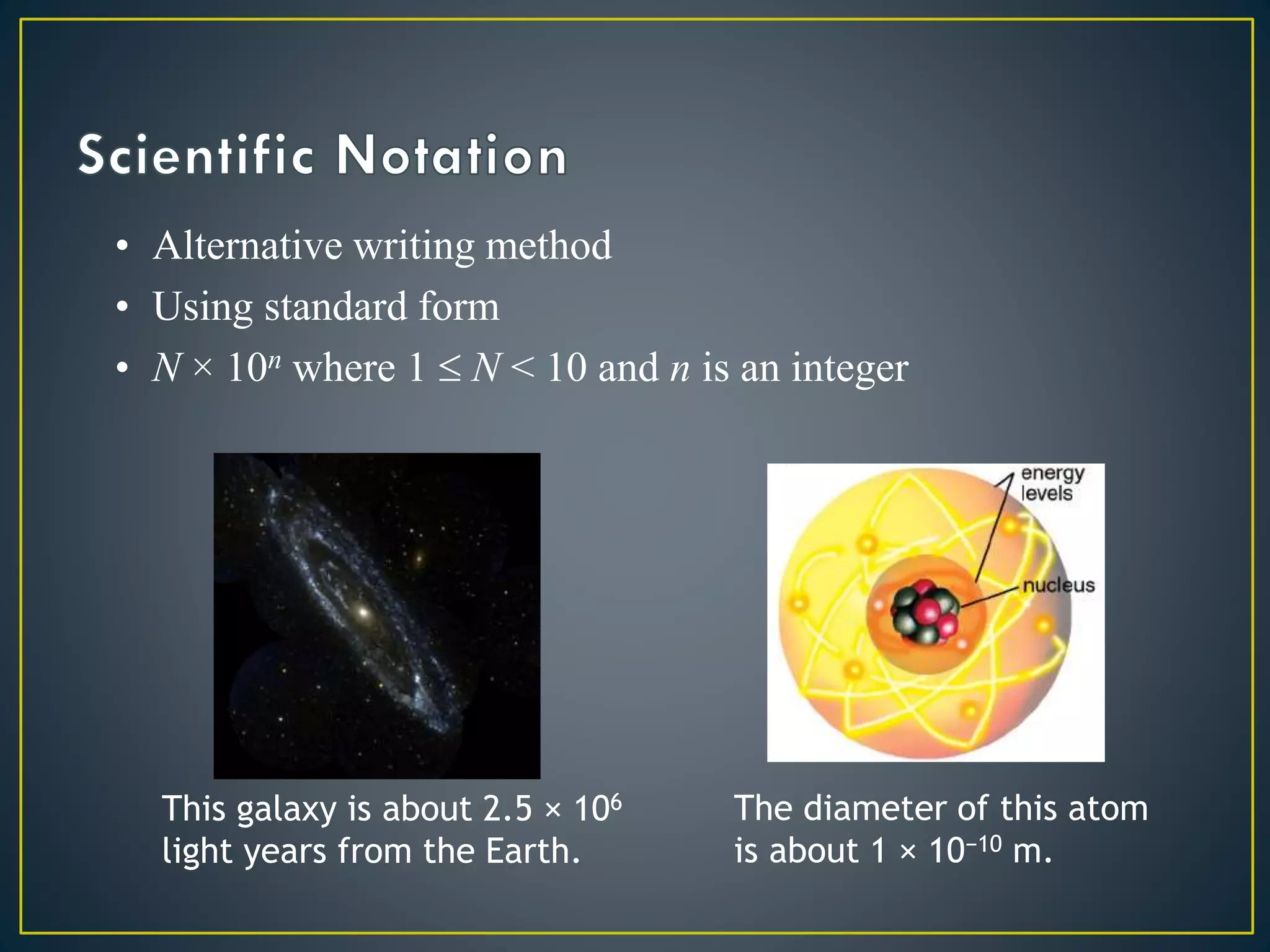
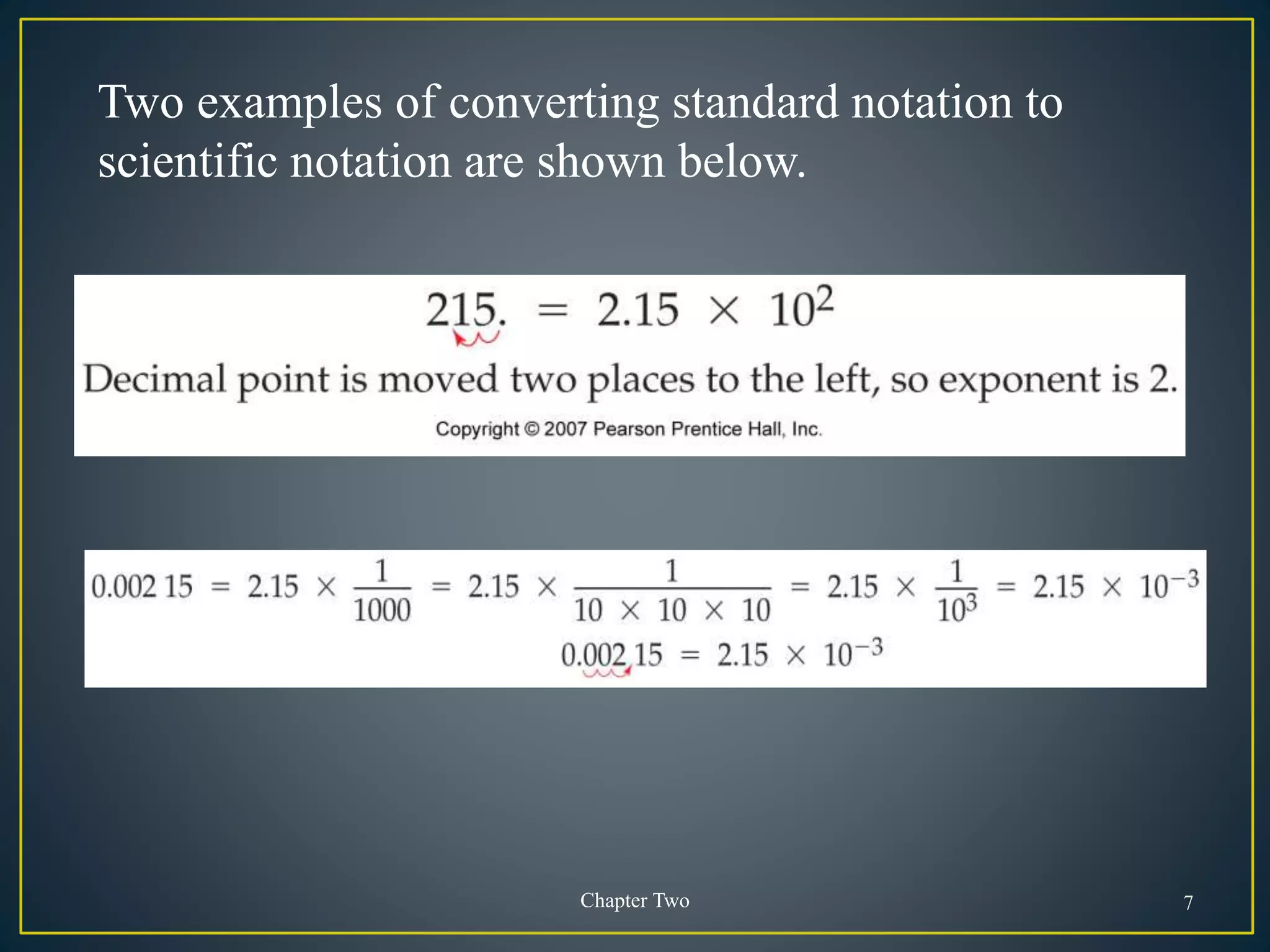
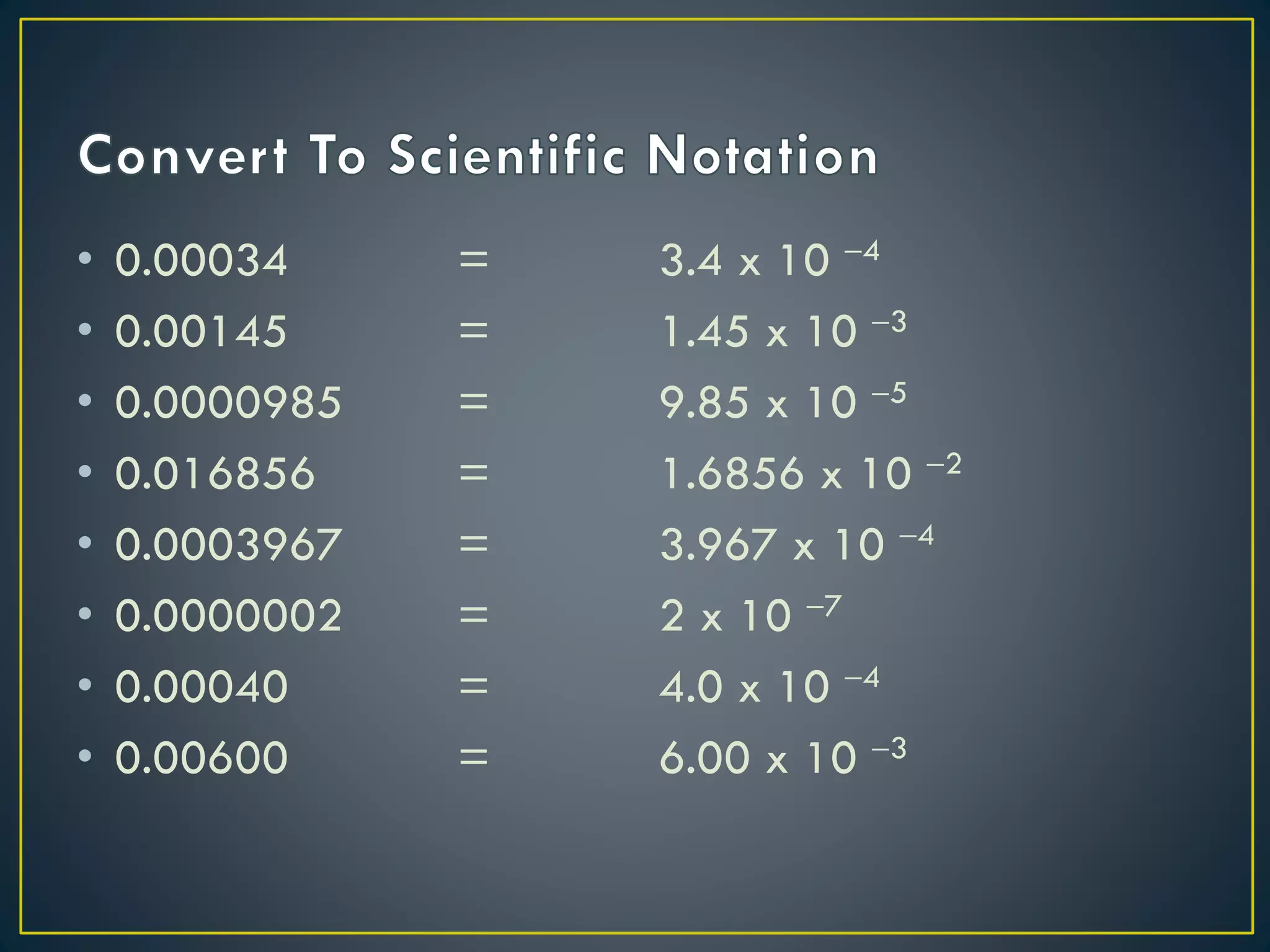
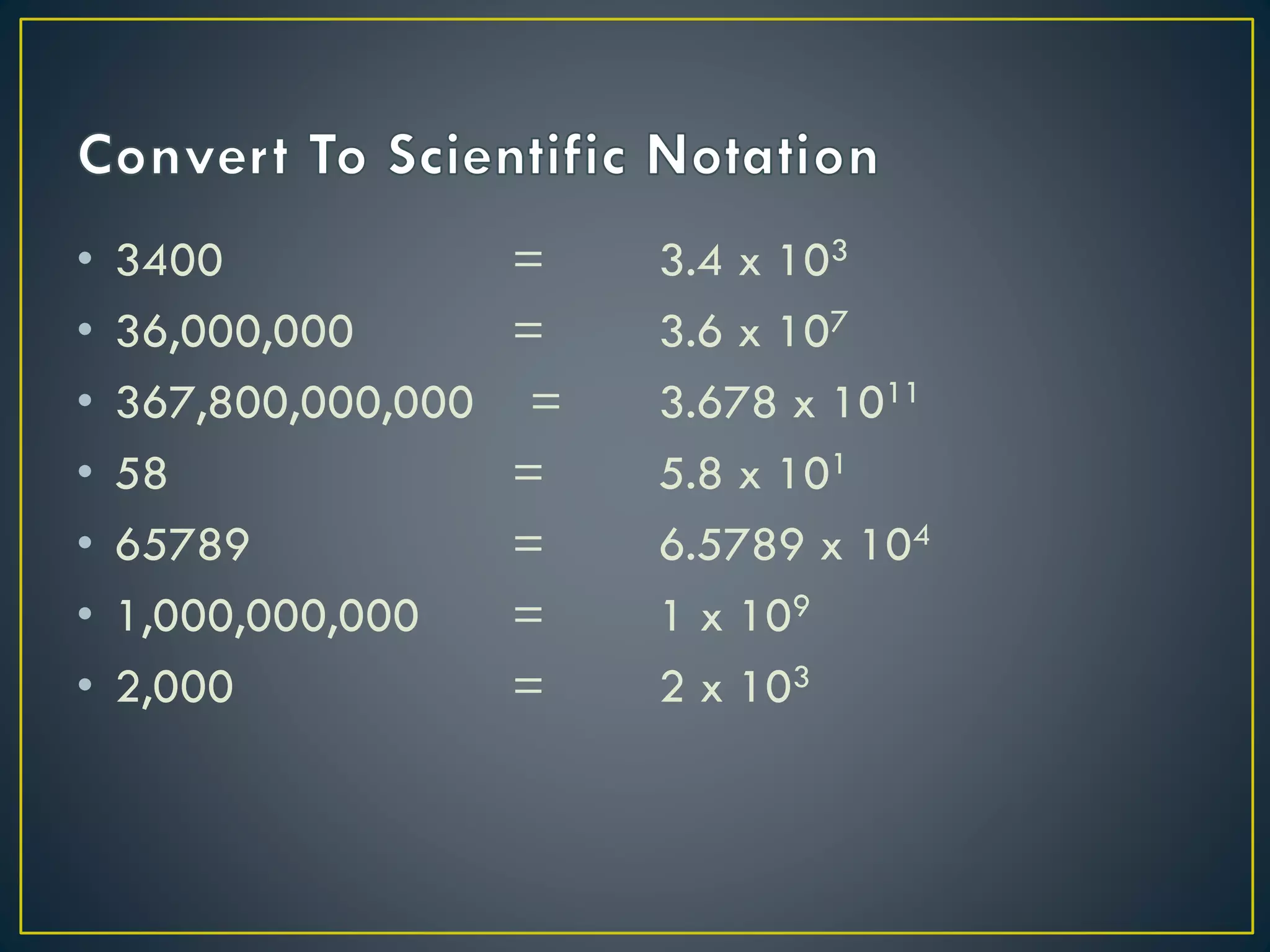
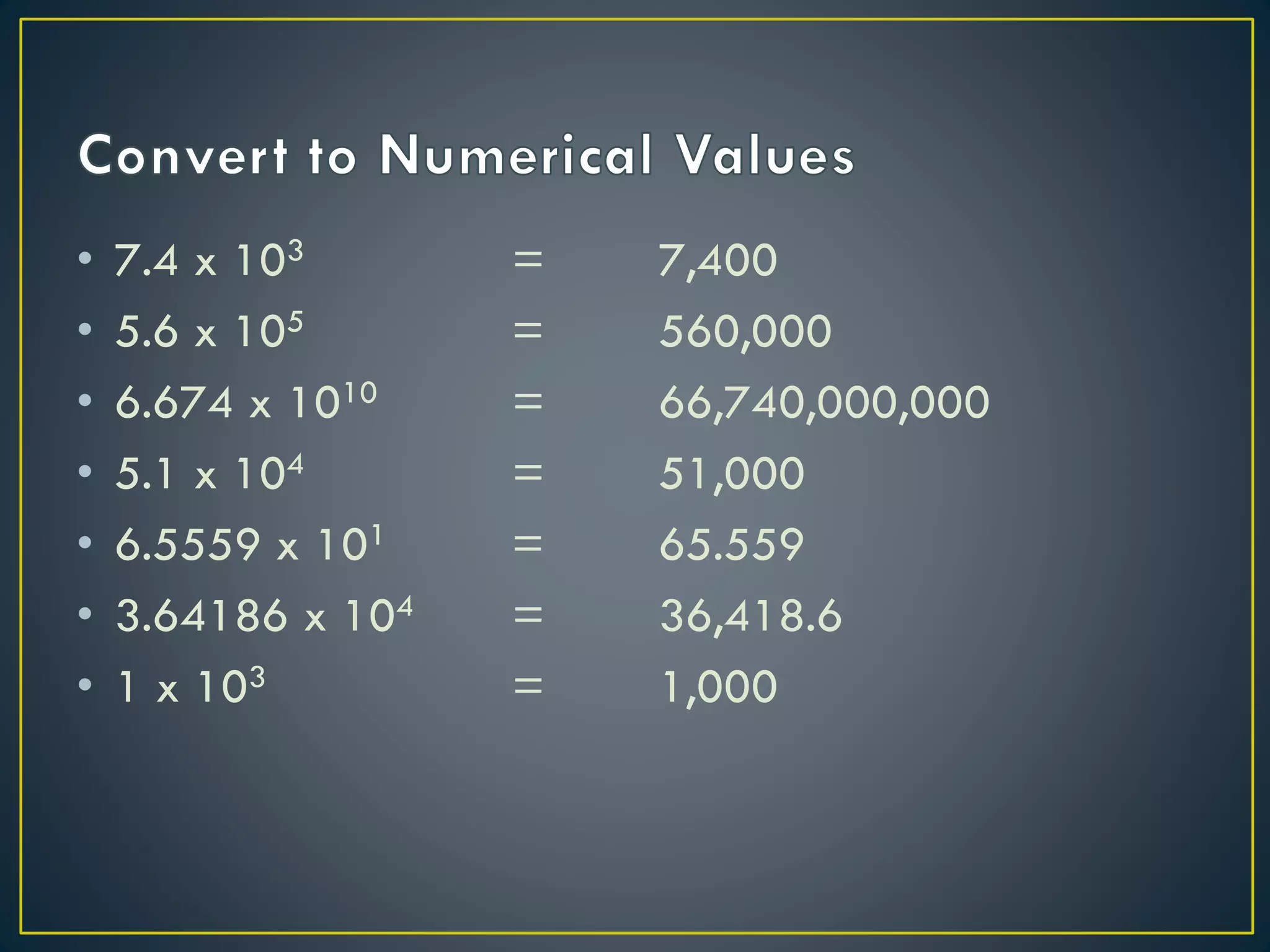
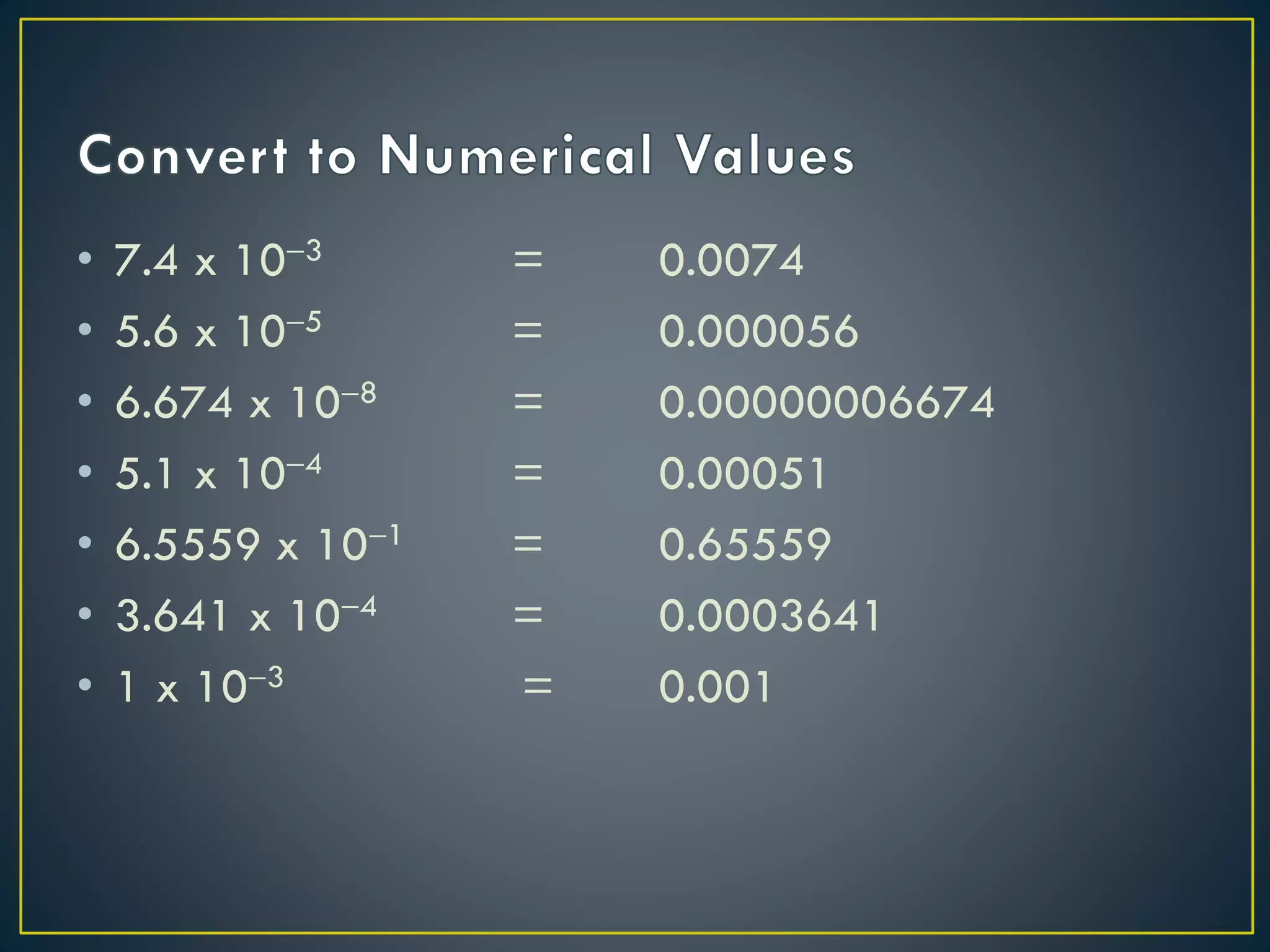
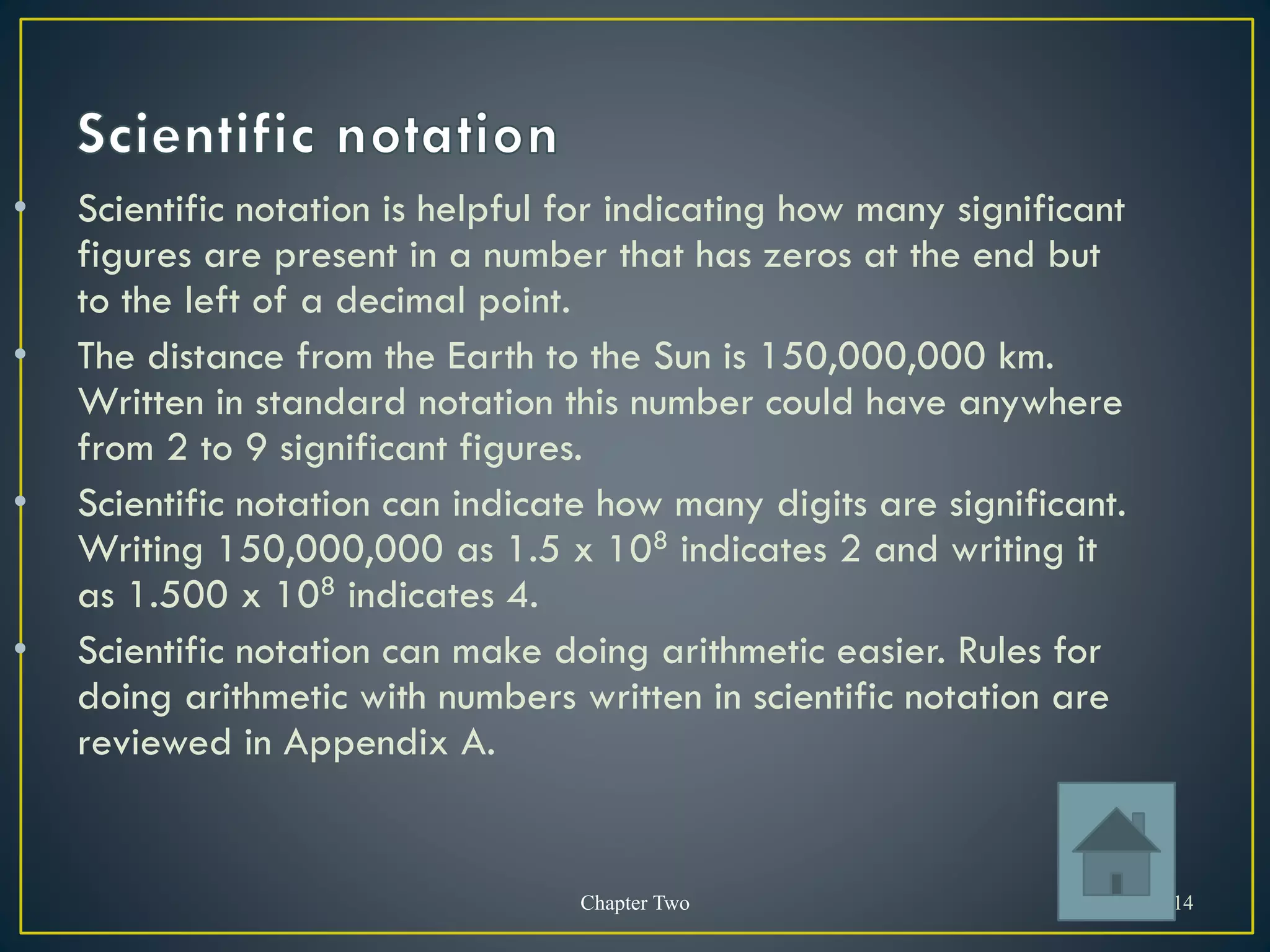
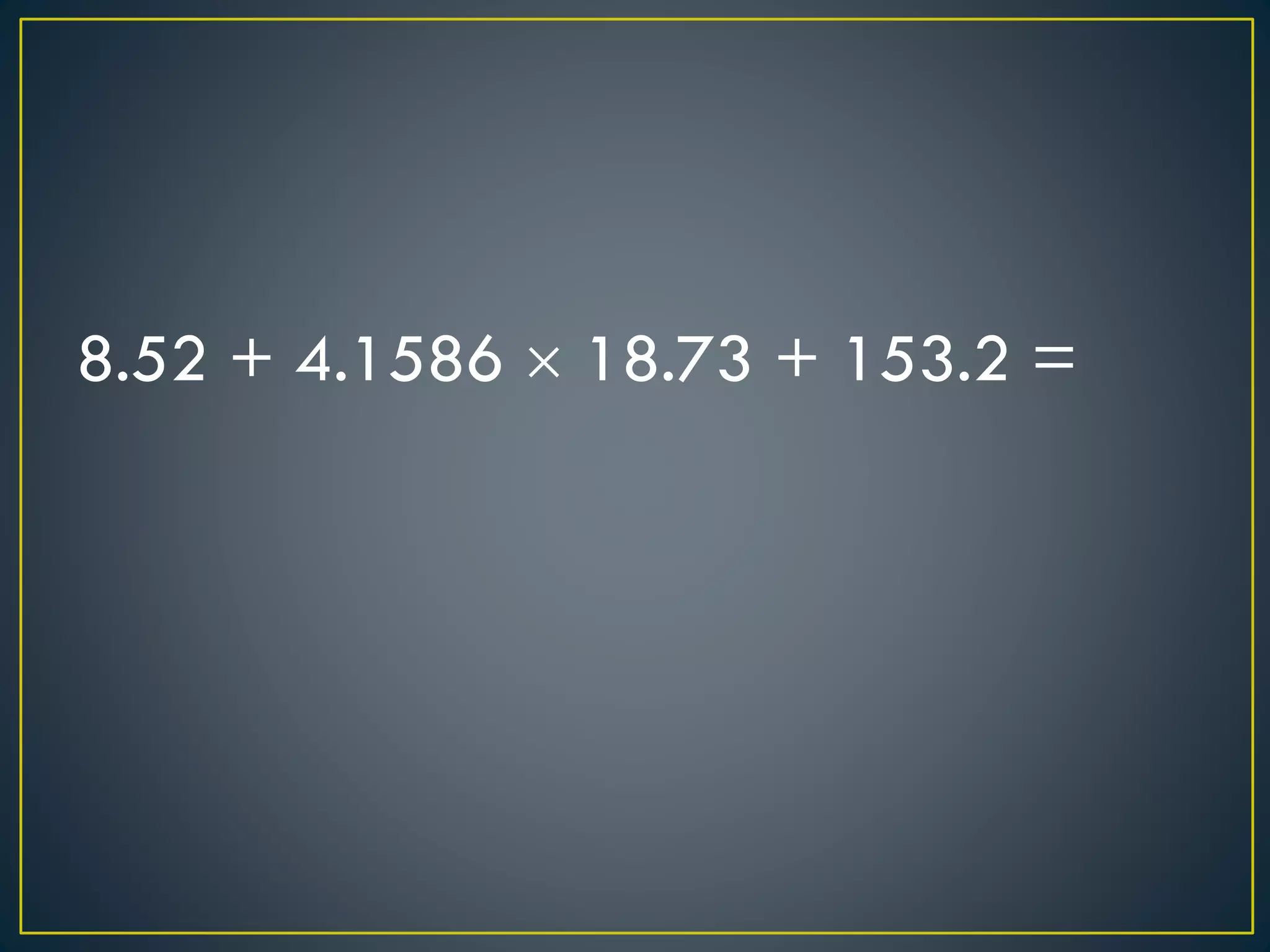- Scientific notation is a way of writing very large or small numbers as a product of a number between 1 and 10 and a power of 10.
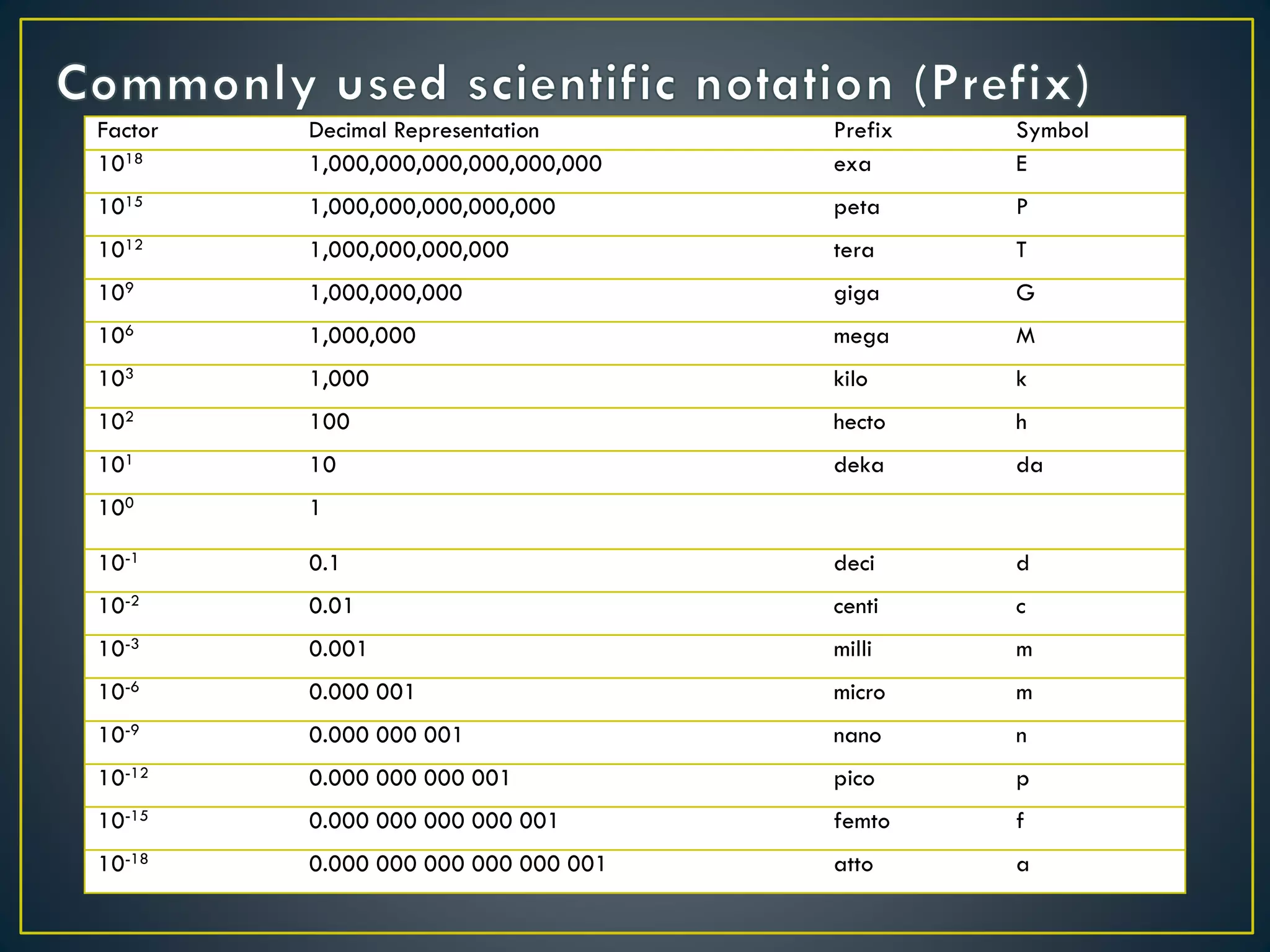
- Prefixes are used to indicate the power of 10 being multiplied, with examples including mega (106) and micro (10-6).
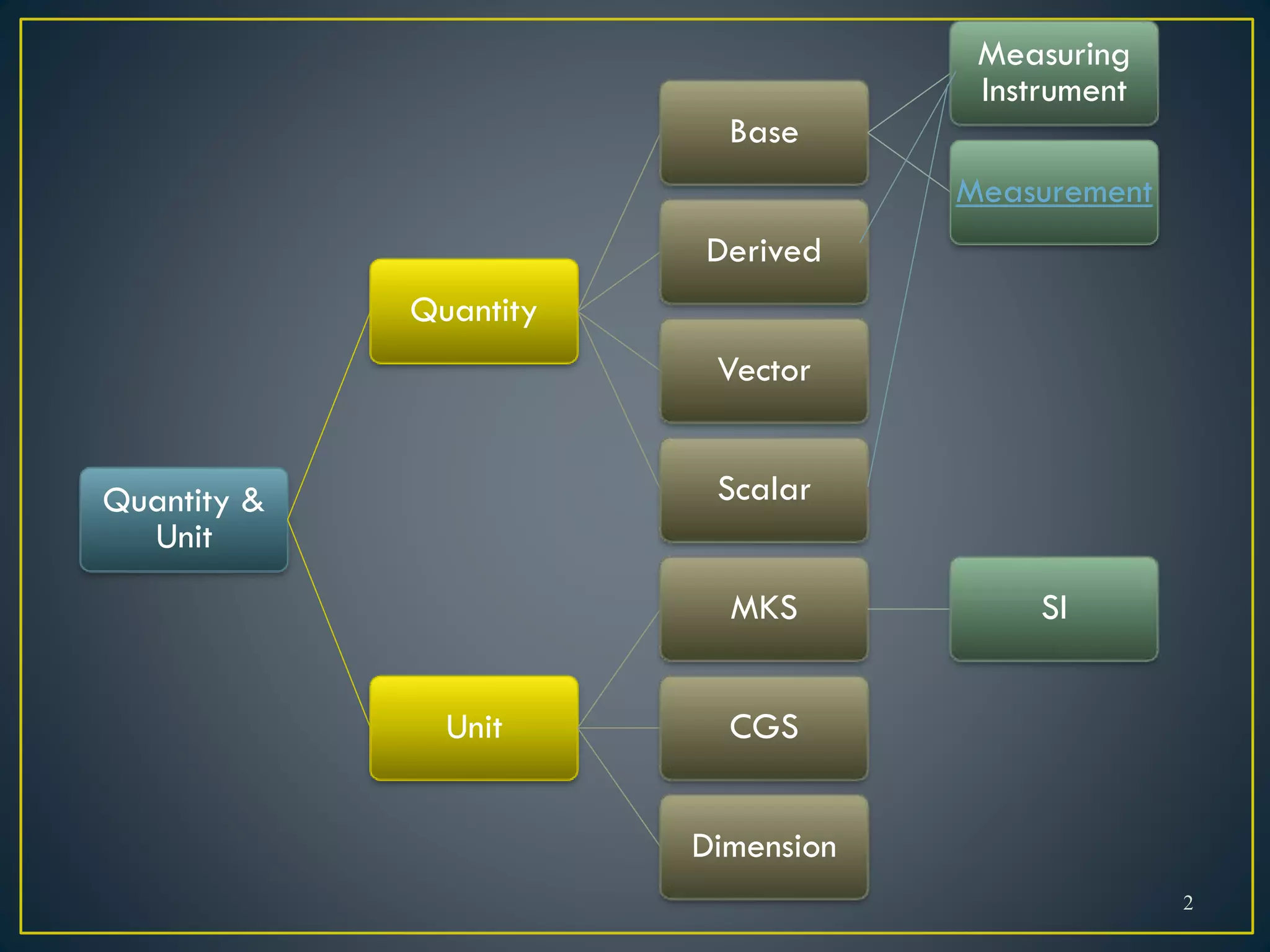
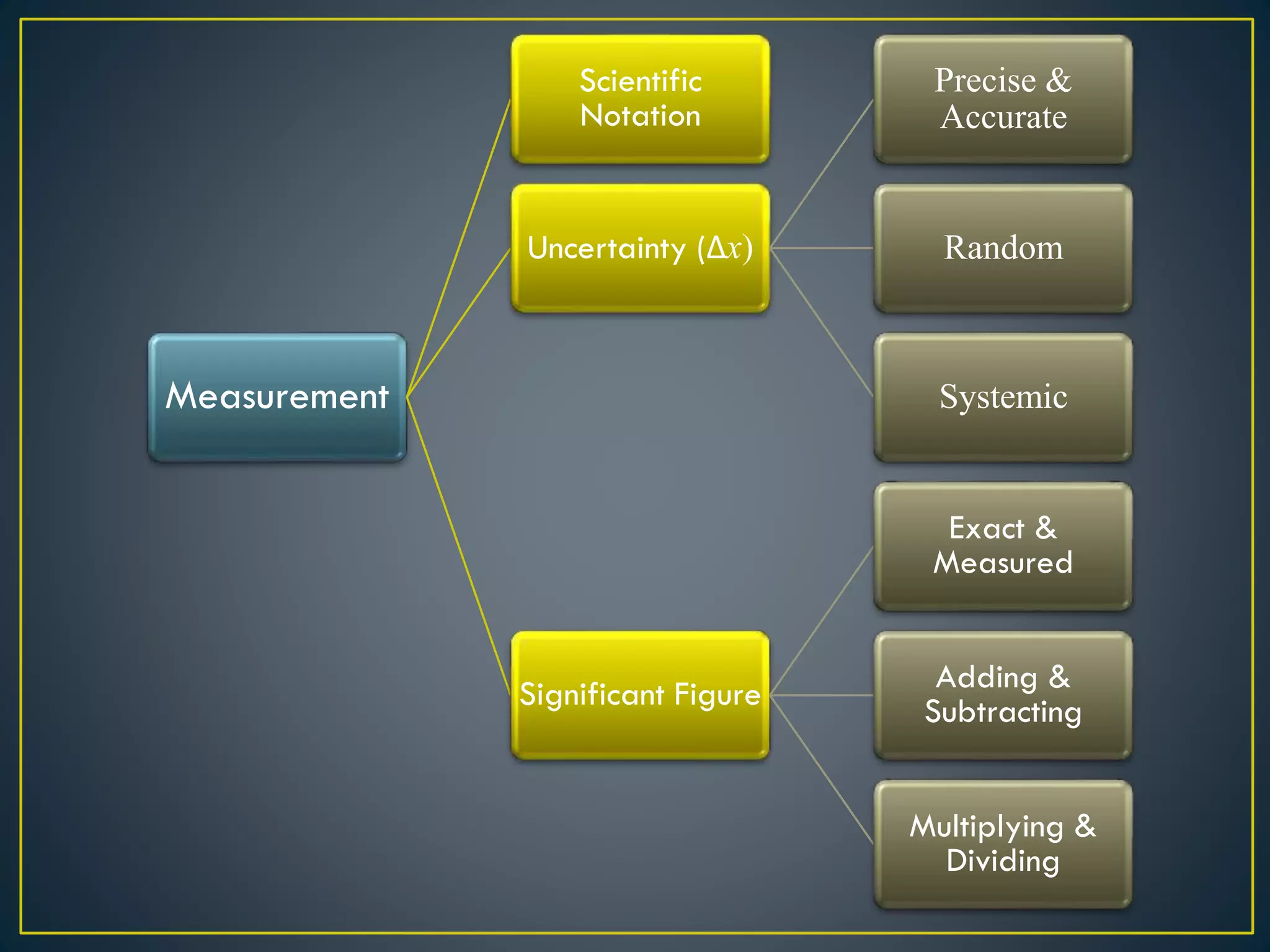
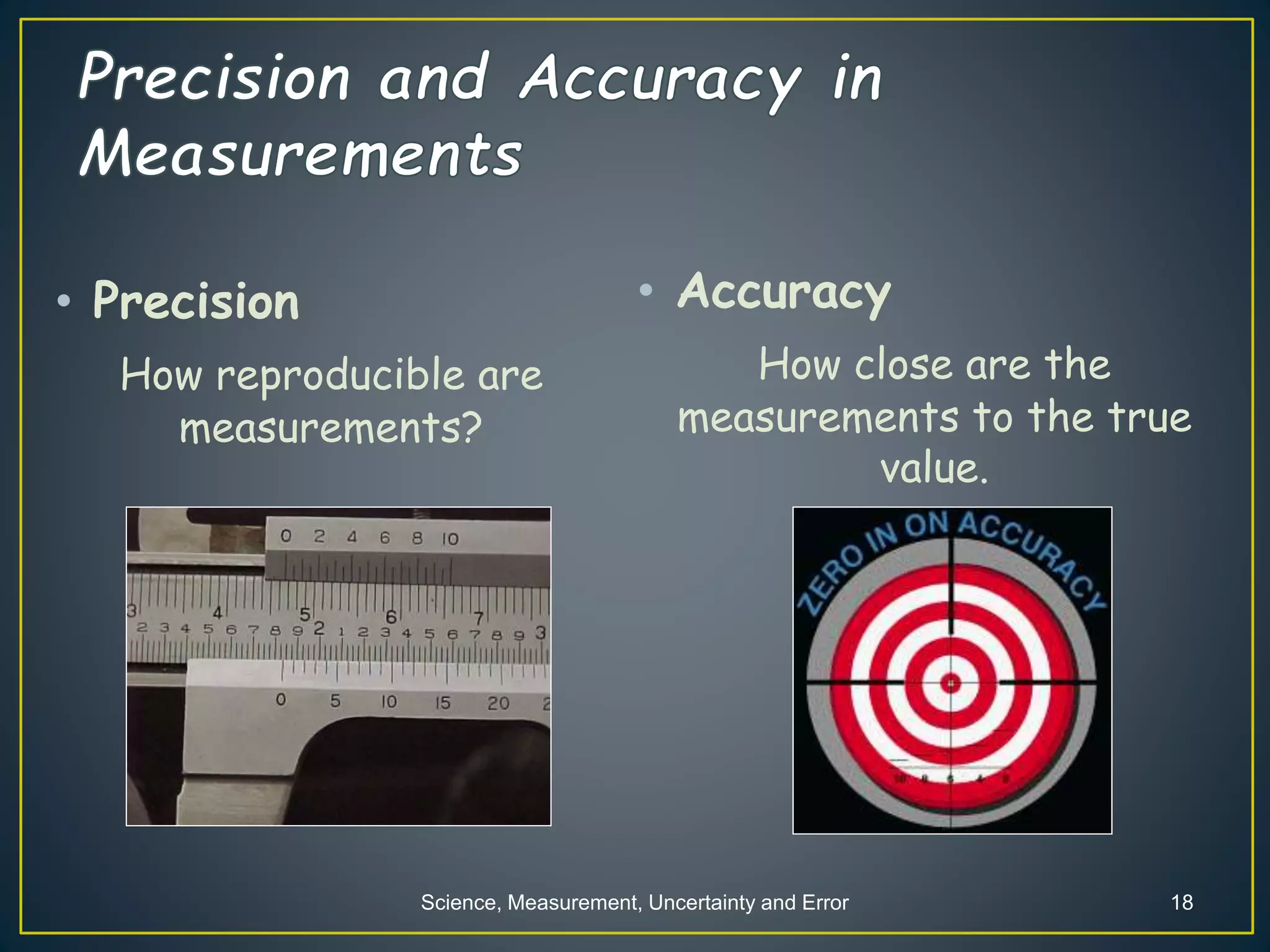
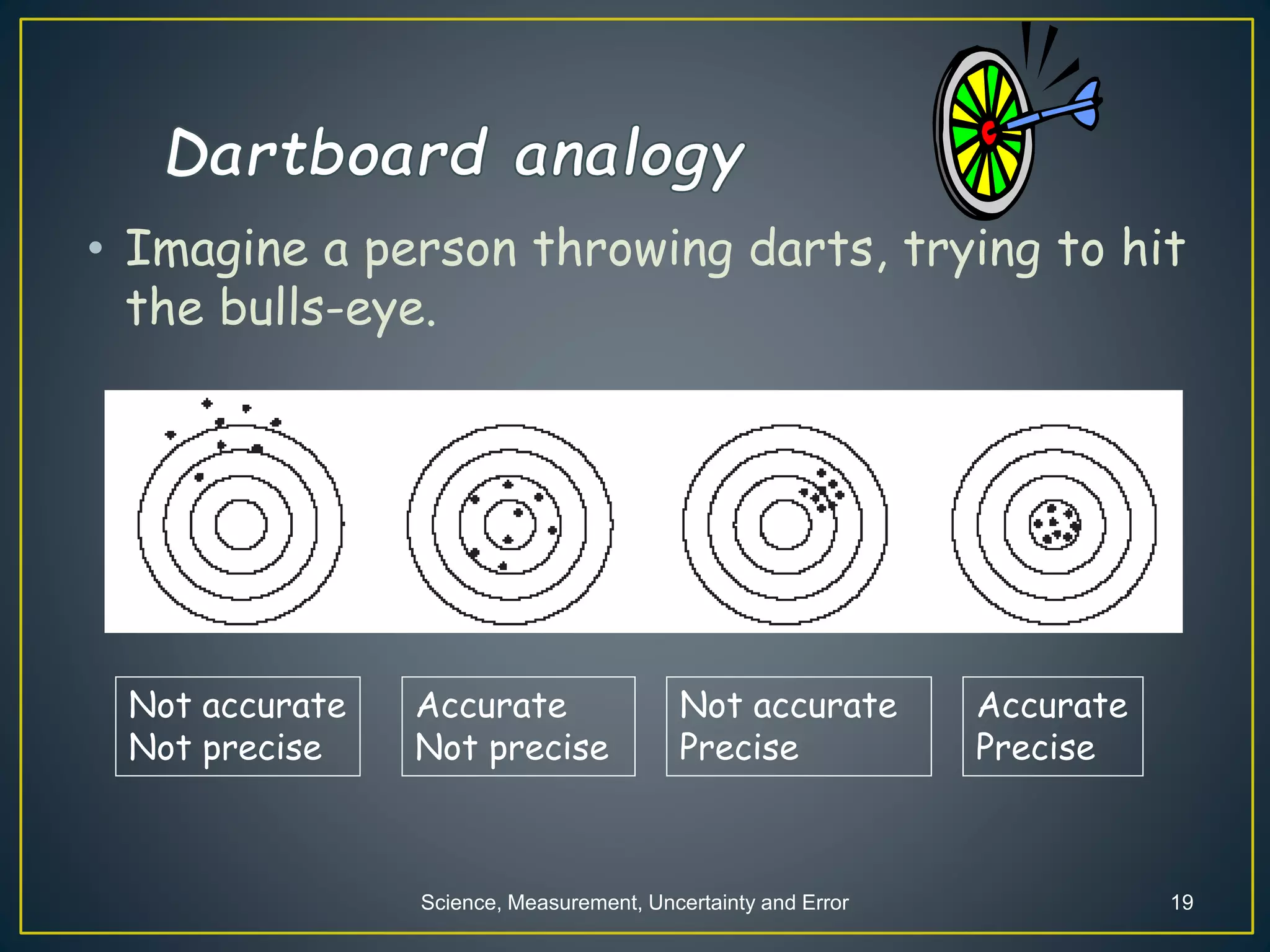
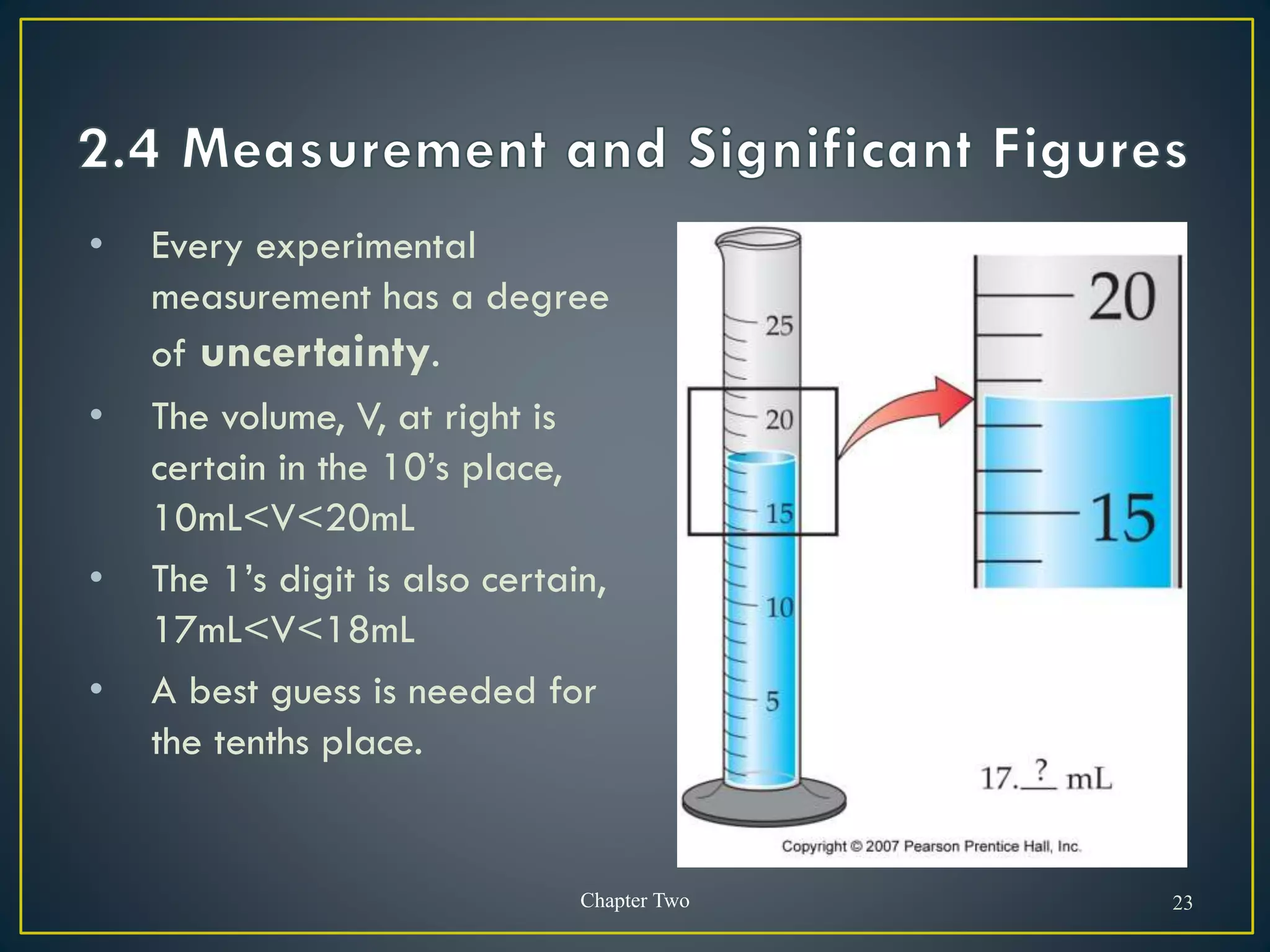
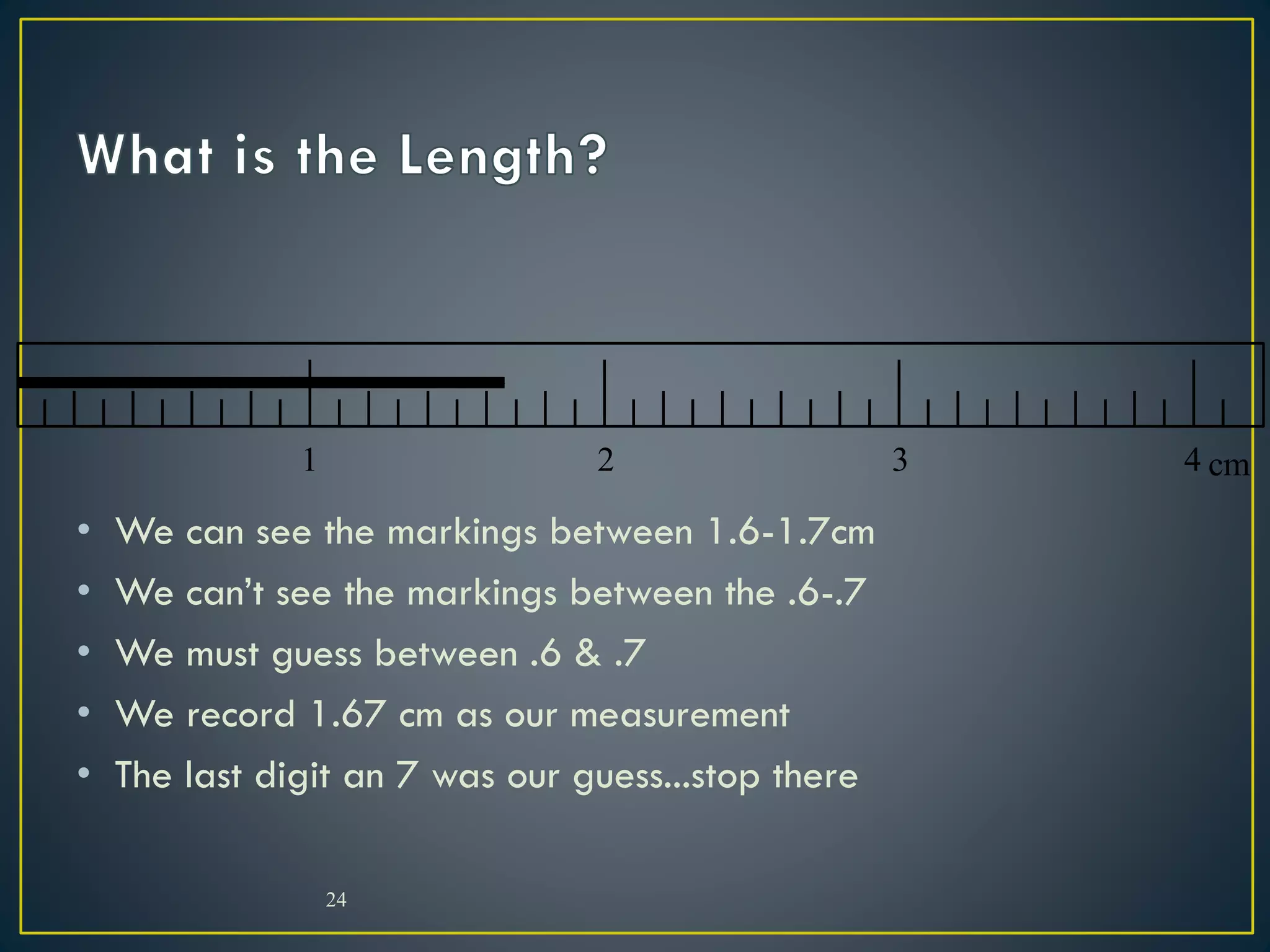
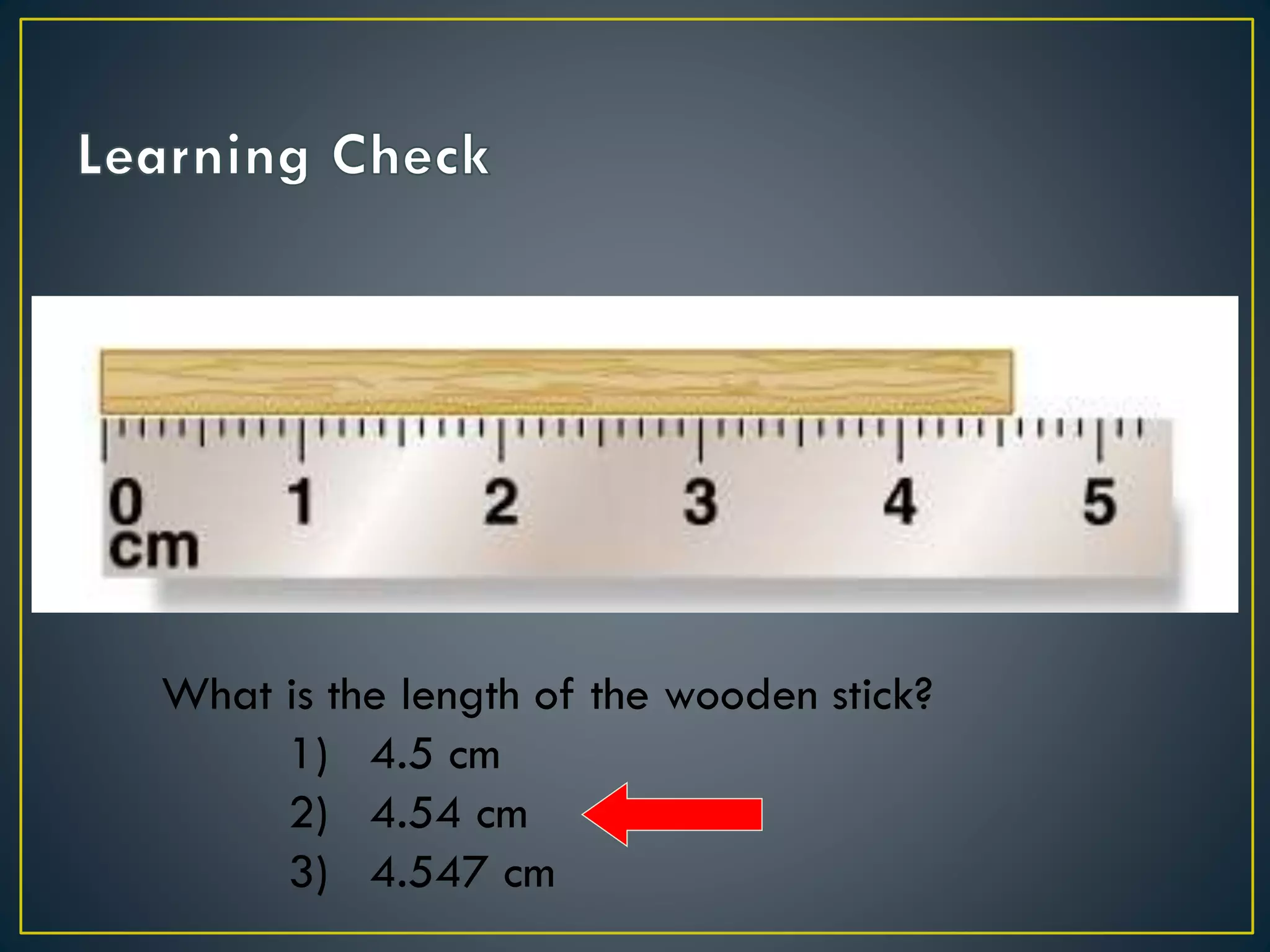
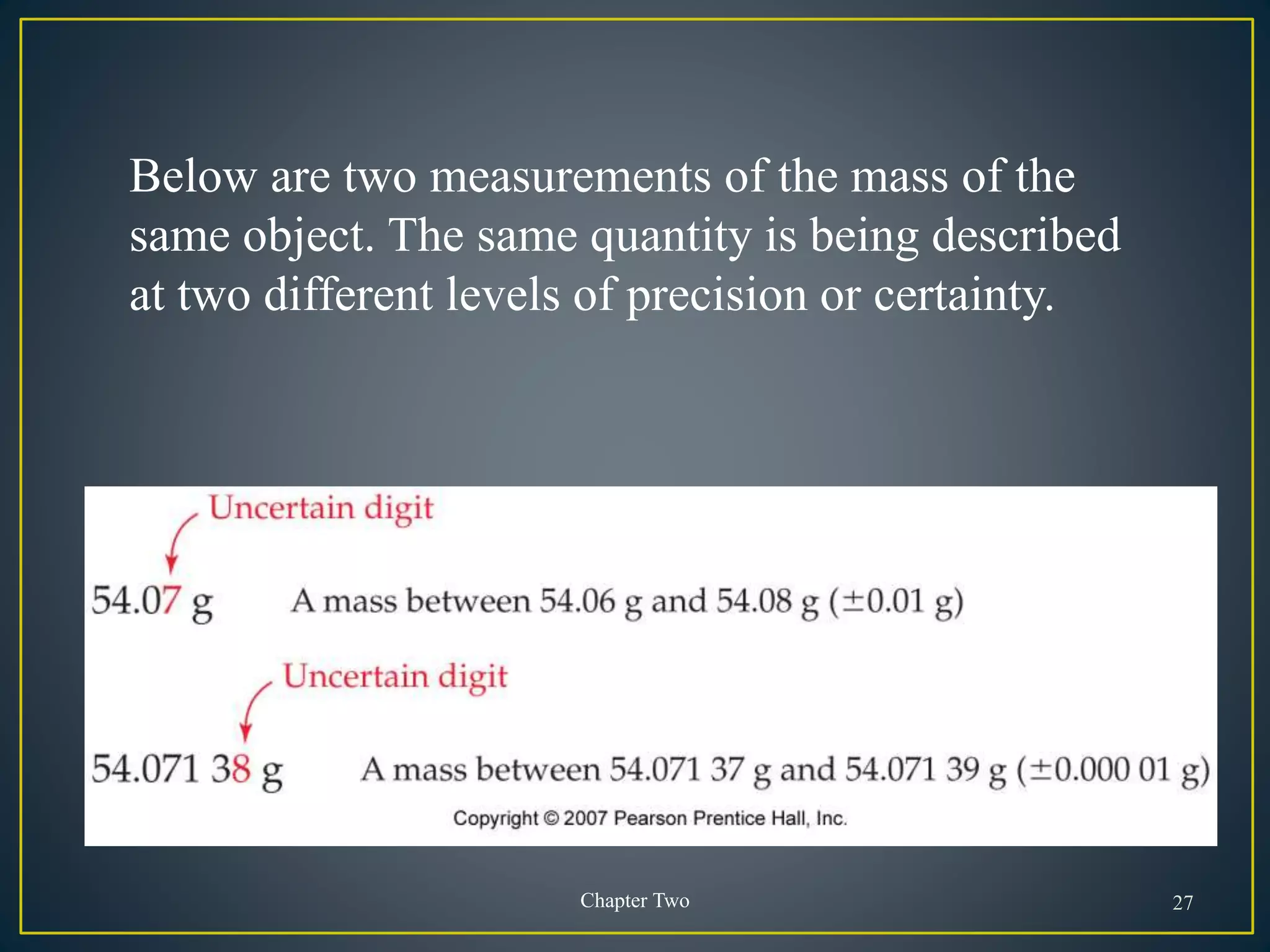
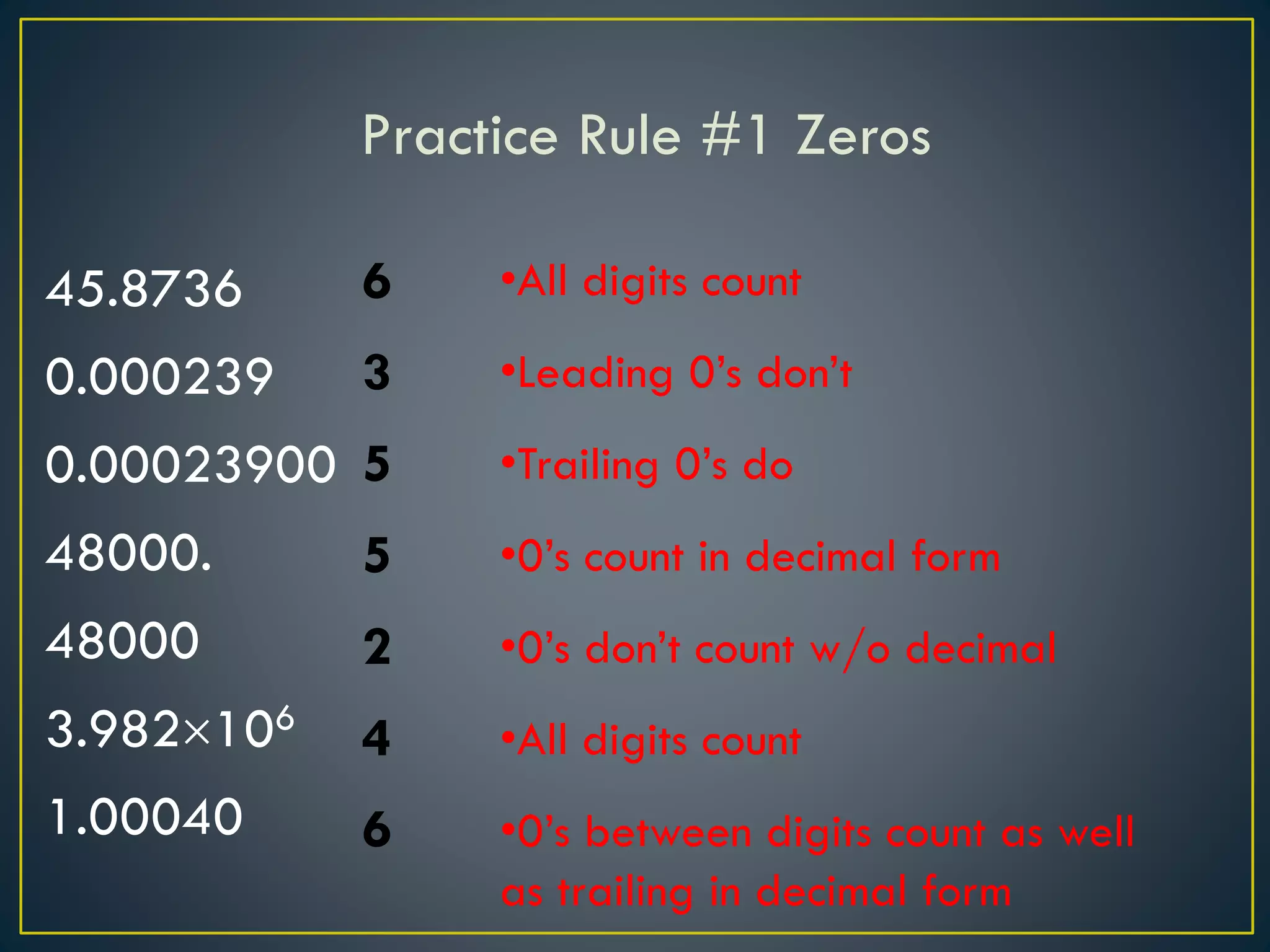
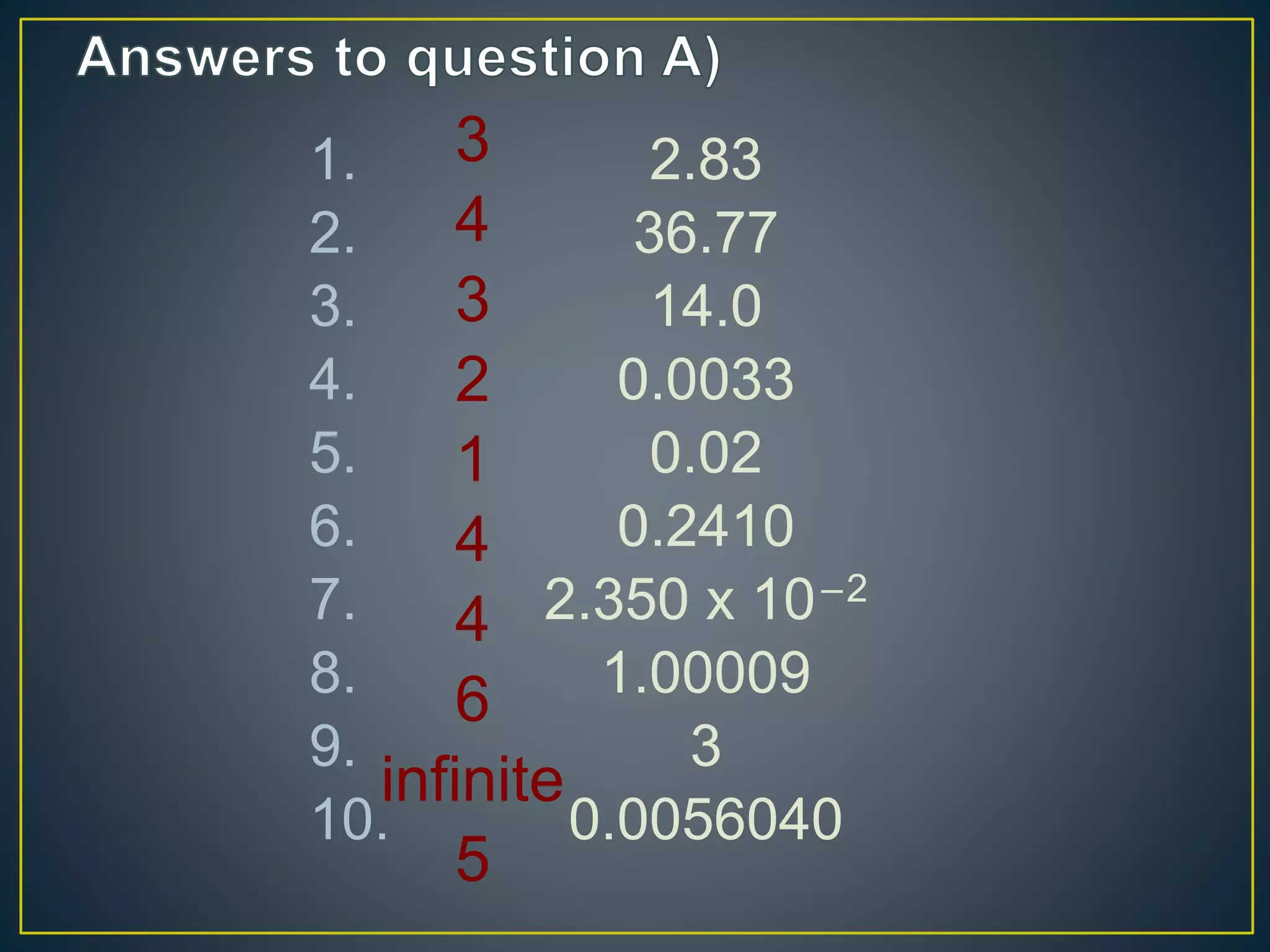
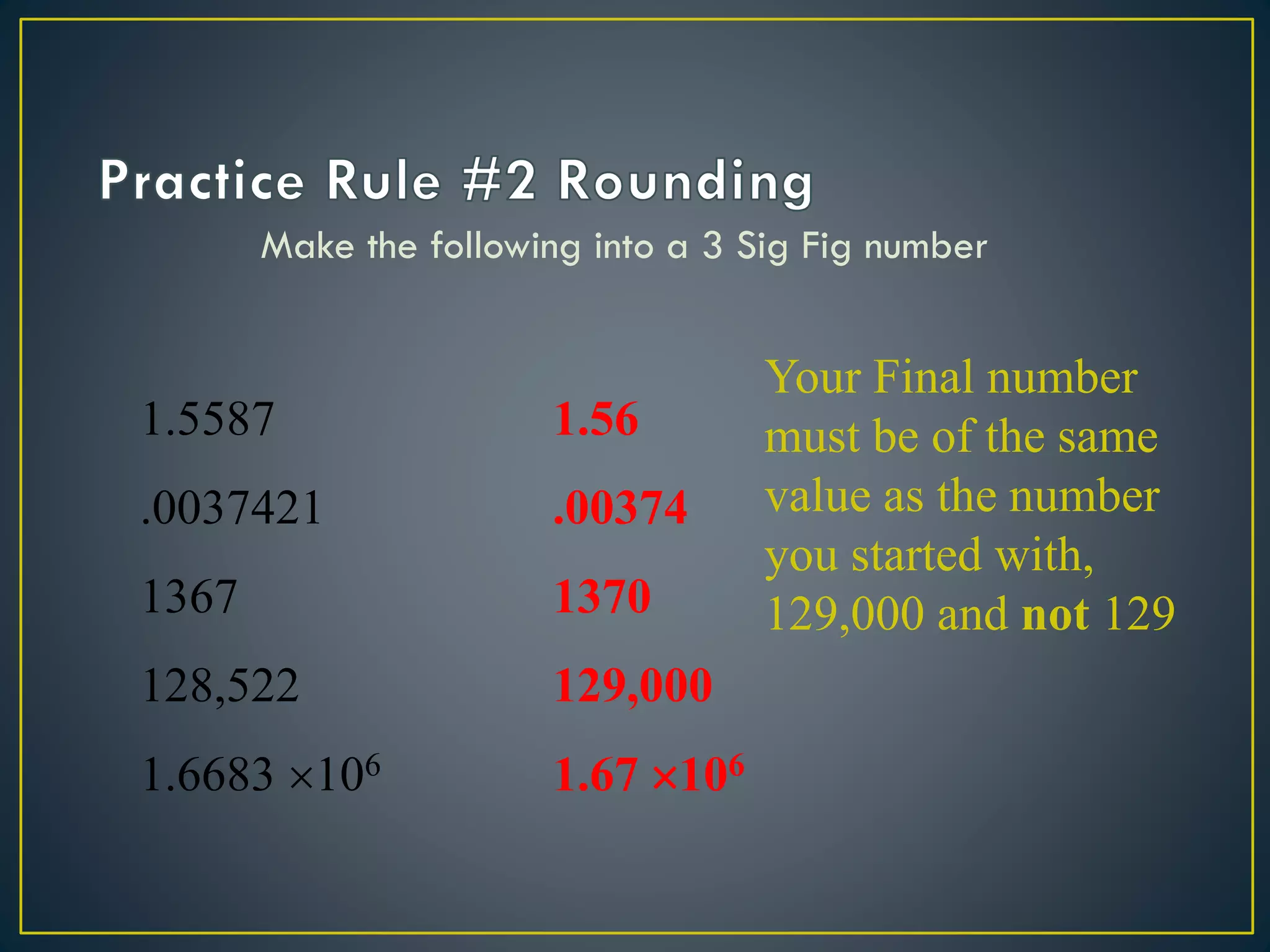
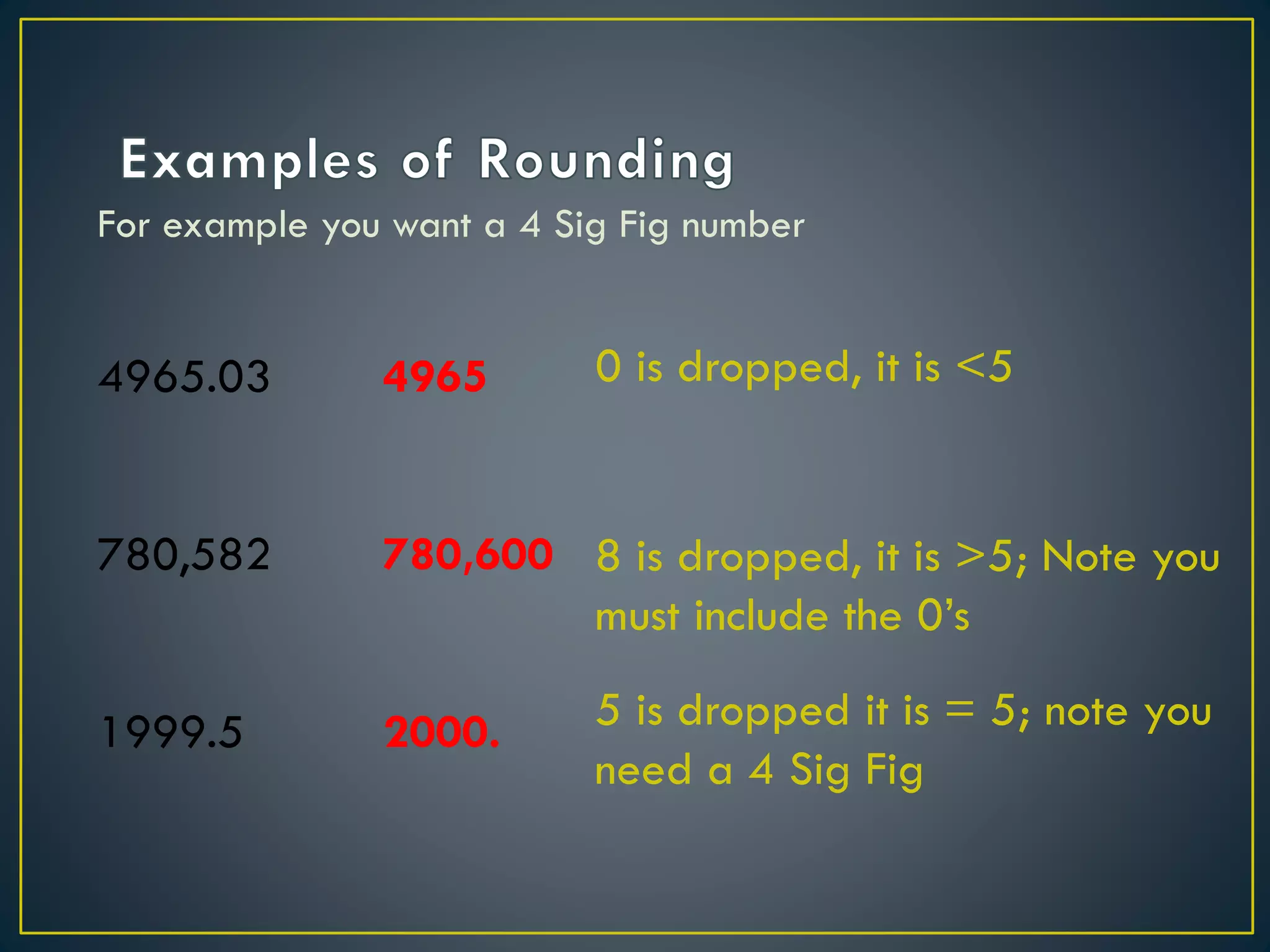
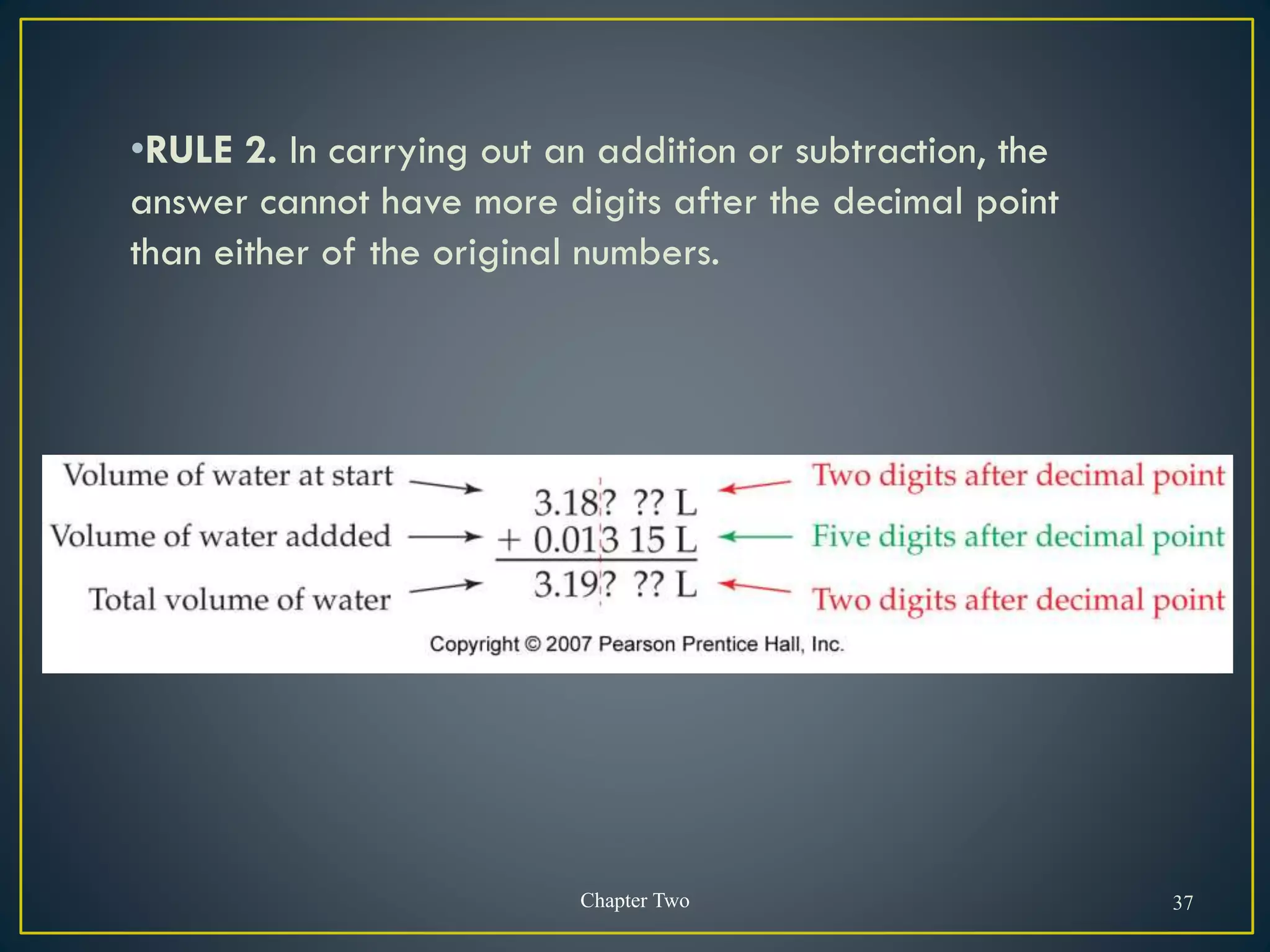
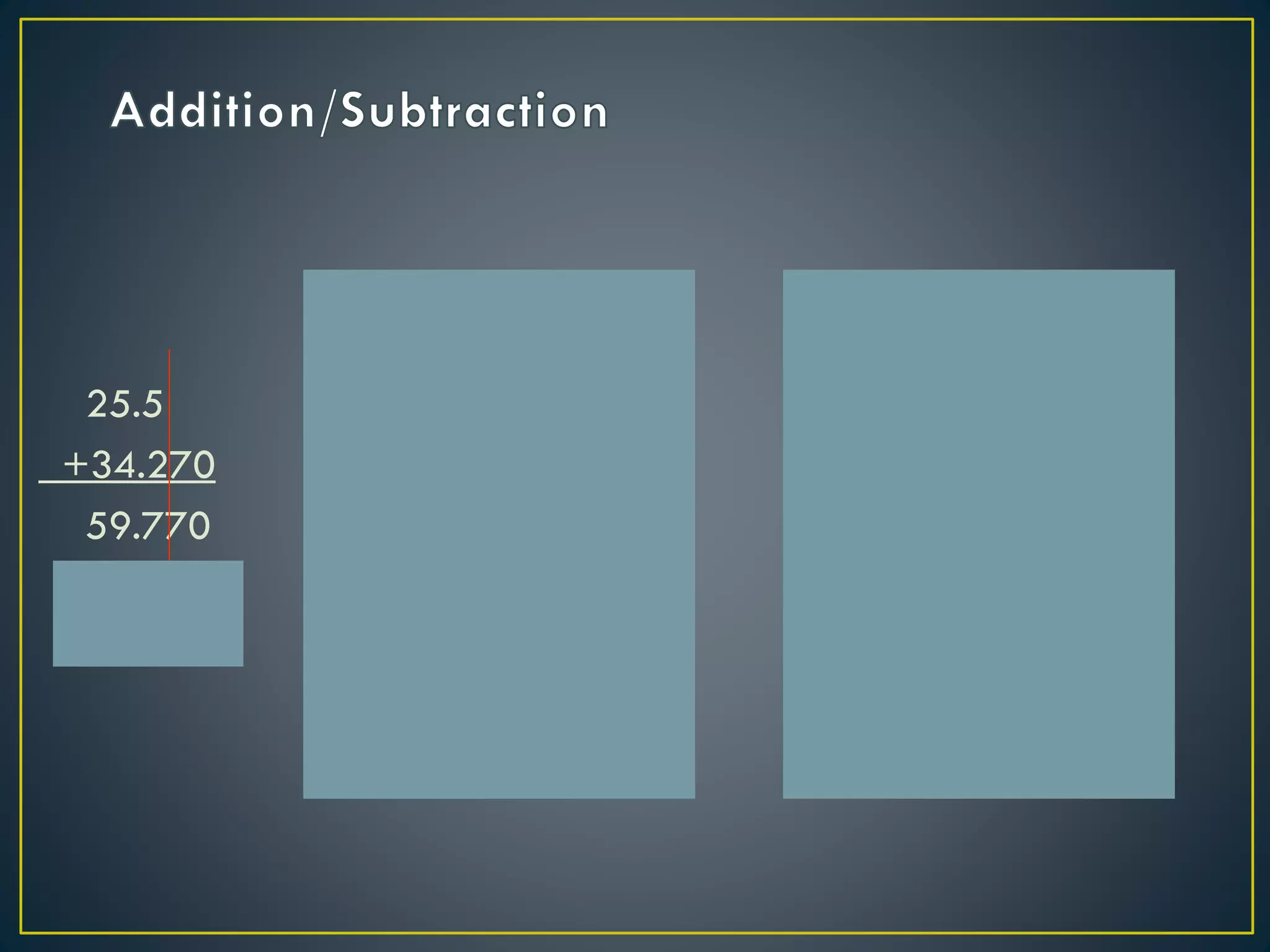
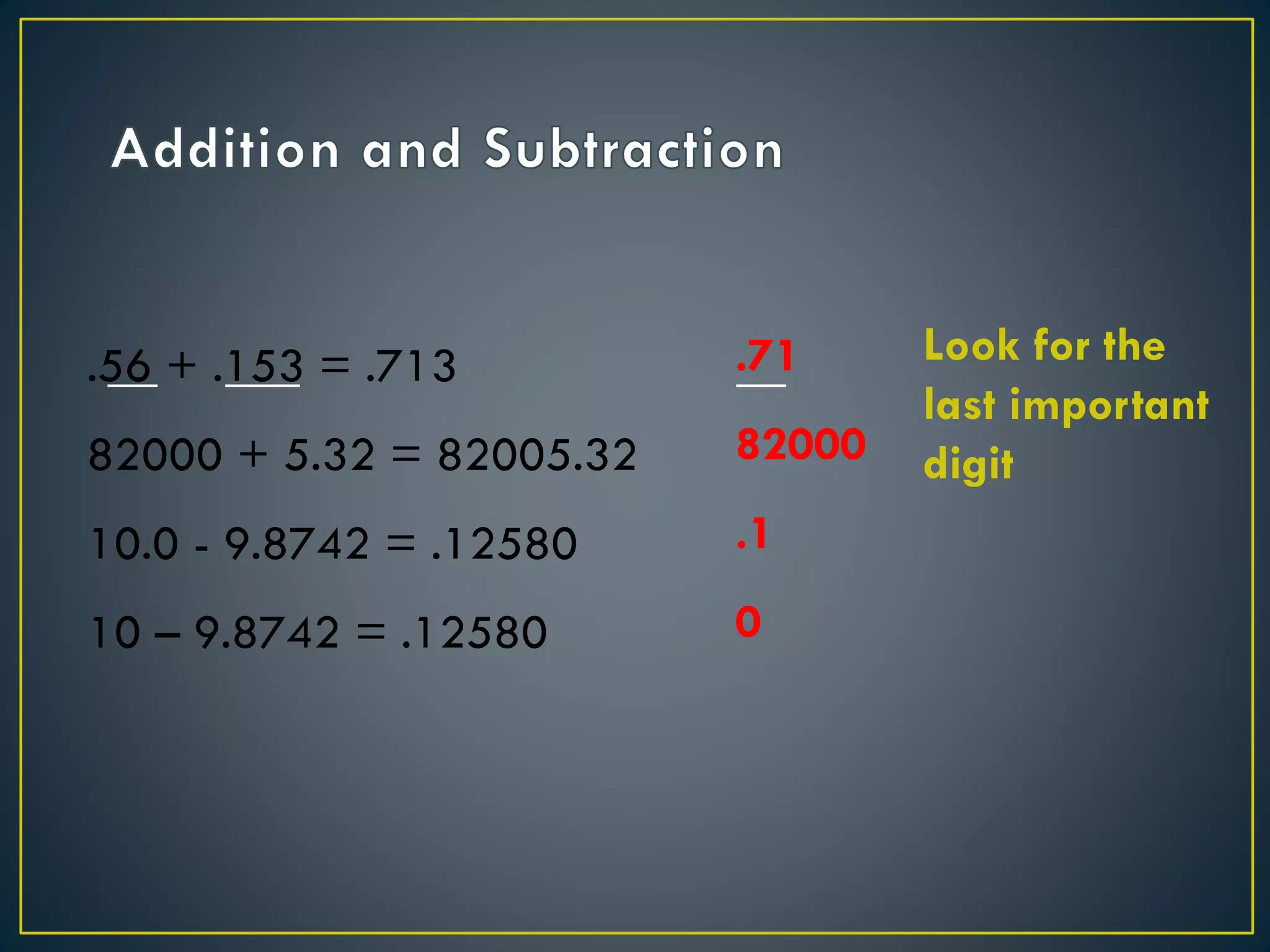
- When measuring quantities, only a certain number of digits can be known with certainty based on the precision of the measuring instrument. Significant figures indicate the digits that are known precisely.